Abstract
Background
Acute brain lesions constitute an alarming public health concern. Neuroprotective therapies have been implemented to stabilize, prevent, or reduce brain lesions, thus improving neurological outcomes and survival rates. Hypothermia is the most effective approach, mainly attributed to the reduction in cellular metabolic activity. Whole-body cooling is currently implemented by healthcare professionals; however, adverse events are frequent, limiting the potential benefits of therapeutic hypothermia. Therefore, selective methods have been developed to reduce adverse events while delivering neuroprotection. Nasopharyngeal approaches are the safest and most effective methods currently considered. Our primary objective was to determine the effects of a novel nasopharyngeal catheter on the brain temperature of pigs.
Methods
In this prospective, non-randomized, interventional experimental trial, 10 crossbred pigs underwent nasopharyngeal cooling for 60 min followed by 15 min of rewarming. Nasopharyngeal catheters were inserted into the left nostril and properly positioned at the nasopharyngeal cavity.
Results
Nasopharyngeal cooling was associated with a decrease in brain temperature, which was more significant in the left cerebral hemisphere (p = 0.01). There was a reduction of 1.47 ± 0.86 °C in the first 5 min (p < 0.001), 2.45 ± 1.03 °C within 10 min (p < 0.001), and 4.45 ± 1.36 °C after 1 h (p < 0.001). The brain-core gradient was 4.57 ± 0.87 °C (p < 0.001). Rectal, esophageal, and pulmonary artery temperatures and brain and systemic hemodynamic parameters, remained stable during the procedure. Following brain cooling, values of oxygen partial pressure in brain tissue significantly decreased. No mucosal lesions were detected during nasal, pharyngeal, or oral inspection after nasopharyngeal catheter removal.
Conclusions
In this study, a novel nasopharyngeal cooling catheter effectively induced and maintained exclusive brain cooling when combined with effective counter-warming methods. Exclusive brain cooling was safe with no device-related local or systemic complications and may be desired in selected patient populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The annual incidence of patients with acute brain lesions (0.44%) constitutes an alarming public health concern worldwide [1,2,3,4]. This high incidence is caused by hypoxic–ischemic brain injury following cardiac arrest as well as severe traumatic brain injury, hemispheric stroke, and high-grade subarachnoid hemorrhage. Primary brain lesions occur during the initial insult and can be aggravated for up to 8 weeks [5, 6]. Secondary brain lesions [7, 8] may occur gradually over subsequent days and worsen primary lesions, imposing a devastating burden on families and societies. Neuroprotective therapies have been implemented with the intention to stabilize primary lesions, prevent or reduce secondary lesions, and improve neurological outcomes and survival rates [9, 10]. Hypothermia is one of the most promising and effective neuroprotective methods, mainly because it reduces cellular metabolic activity [11,12,13]; however, other mechanisms [14,15,16,17] also contribute to the observed benefits. Therapeutic hypothermia and targeted temperature management have been recommended by the American Heart Association [18,19,20] and The Cochrane Collaboration [21] for preserving cerebral function of patients surviving resuscitation after cardiac arrest. The indication for therapeutic hypothermia in patients with traumatic brain injury is still controversial, but recent meta-analyses have suggested that this method is beneficial [22, 23].
Whole-body cooling is the current method implemented by healthcare professionals; however, adverse events are frequent with incidences of cardiovascular and infectious complications that vary from 34 to 52% [21, 24,25,26], which limits the potential benefits of therapeutic hypothermia. Therefore, more selective methods have been developed and tested with the aim of reducing adverse events while delivering neuroprotection, with fewer risks to patients [5, 21]. Among the methods with preferential action on brain tissue, nasopharyngeal approaches are the safest and most effective currently under study [27,28,29,30,31,32]. Their anatomical positioning facilitates heat exchange with cervical blood vessels and cerebrospinal fluid flow directed to the brain. Furthermore, conduction of heat through the skull base bones is also effective. Perfluorocarbon gas [27, 29, 30], oxygen [31], and compressed air [32,33,34,35] administration into the nasopharyngeal cavities is able to cool the brain, but these methods do not allow precise control of temperature, and safety issues remain [36]. Methods utilizing circulating water are the most promising approaches due to their superior safety profile and ability to exchange heat with nasopharyngeal surfaces effectively [37]. However, no nasopharyngeal method has yet proven whether brain cooling without systemic temperature reduction is possible and assessed whether this approach could reduce the systemic consequences of core temperature reduction and its related adverse events.
Our primary objective was to determine the effects of a novel nasopharyngeal catheter on the brain temperature of pigs. The secondary objective was to assess the local effects of the nasopharyngeal cooling catheter and systemic effects of exclusive brain cooling.
Methods
This prospective, non-randomized, interventional experimental trial was conducted to investigate the feasibility of inducing exclusive brain cooling during a period of 60 min followed by 15 min of rewarming. Counter-warming was implemented to prevent core temperature reduction using blankets and thermal mattresses during the intervention.
The study protocol was submitted to and approved by the Institutional Animal Care and Use Committee of the School of Medicine at the University of São Paulo (USP) (Approval number: 1.085/09). The study was developed according to the recommendations of the National Institute of Health [38], American Physiological Society [39], National Council for the Control of Animal Experimentation [40], and the Ethics Committee on Animal Use [41]. All applicable institutional and national guidelines for the care and use of animals were followed. Experimental animals were purchased from breeders near São Paulo, following the legal procedures of the Department of Agriculture and Supply and Agricultural Defense, and were delivered to the School of Veterinary Medicine and Animal Science at the USP, such that they could be sent to the School of Medicine at USP on the morning of the trial.
Inclusion Criteria
Research subjects were eligible if they were the following: [1] healthy crossbred pigs; [2] of either sex; [3] aged 3–6 months; [4] weighed between 15 to 25 kg; and [5] had baseline arterial oxygen saturation above 92%.
Exclusion Criteria
Research subjects were ineligible if they had the following: [1] baseline brain temperature below 36 °C; [2] anatomical obstructions of the upper airway; [3] current participation in another ongoing experiment; [4] hemodynamic instability; and [5] inadequate anesthetic level.
Study Interventions
Preparation
Animals (Landrace, Duroc, and Pietrain; 50% male) had free access to food and water the night before the experiment. Pre-anesthesia was initiated 30 min before any procedure and consisted of an intramuscular injection of ketamine (15 mg/kg) and xylazine (2 mg/kg) [42]. The marginal ear vein was punctured with a 20G catheter (BD Insyte Autoguard; Franklin Lakes, NJ, USA). Anesthesia was induced with intravenous administration of sodium thiopental (10 mg/kg) and maintained with the same substance (180 µg/kg/min), along with fentanyl citrate (0.08 µg/kg/min) [42]. Tracheostomy was performed, and an endotracheal tube (Portex; Smiths Medical, Minneapolis, Minnesota, USA) with a diameter of 6 mm was inserted for safe airway assessment. Volume-controlled mechanical ventilation was implemented using a microprocessor-equipped anesthesia ventilator (DX 5010; Philips/Dixtal, Manaus, Amazonas, Brazil). The minute-volume was adjusted to keep the partial pressure of end-tidal CO2 between 35 and 45 mmHg. Positive end-expiratory pressure was maintained at 5 mmHg, and the fraction of inspired oxygen was 100%. Arterial blood samples were collected to calibrate capnography and to confirm pre-established baseline variables. Animals were hydrated with 250 mL of saline solution before initiating experimental procedures and underwent a 30-min period of respiratory and hemodynamic stabilization prior to the start of the main intervention.
Surgical Implantation of Intracranial Pressure Catheters with a Thermometer and Partial Pressure of Oxygen (PbtO2) Sensor
An intraparenchymal intracranial pressure (ICP) catheter coupled to a thermometer (Neurovent-P-T; Raumedic, Helmbrechts, Germany) was implanted in the right hemisphere. A second ICP catheter with a thermometer and PbtO2 sensor (Neurovent-P-TO; Raumedic) was implanted in the left hemisphere. The catheters were inserted through cranial burr holes positioned 0.5 cm lateral to the midline and 1 cm above the coronal suture bilaterally, and their tips were positioned 1.5 cm below the cortical surface. Intraoperative ultrasonography was used to confirm the position of the ICP catheter tip in the cortical parenchyma.
Nasopharyngeal Brain Cooling and Counter-Warming
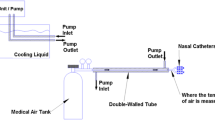
Brain cooling was induced and maintained with a nasopharyngeal cooling catheter (Fig. 1) inserted into the left nostrils of all animals and externalized through the oral cavity. The catheter consisted of a tube with two non-expandable sections; a nasal (proximal) section with an internal diameter of 1/4” and an oral (distal) section with an internal diameter of 3/8”. Between the two non-expandable sections, the catheter contained a semi-expandable section with a thin wall expected to expand to 5 cm in diameter and conform to the nasopharyngeal cavity under the pressure of 12 psi generated by a water pump. A small portion of the semi-expandable section was observed in the oropharynx to confirm the proper position of the catheter and prevent it from occupying laryngeal or oral cavities. The final position of the cooling catheter included the left nostril, nasopharynx, oropharynx, and oral cavity.
The catheter was then connected to an insulated reservoir containing 1 L of water and 1 kg of ice in a closed loop arrangement under continuous flow provided by a submersible centrifugal water pump (S180; Sarlo Better, São Paulo, SP, Brazil) with inflow through the proximal section and outflow through the distal section (Fig. 2). Cold water was maintained at a temperature range between 0 and 2 °C, while ice was replaced when water temperatures exceeded this range, and the flow rate was set to 1–3 L/min. During the cooling intervention, counter-warming was employed with thermal mattresses (150 W) and blankets and maintained for an additional 15 min during the rewarming phase. Brain temperature reduction was targeted to differ by at least 1 °C from baseline temperature without a lower temperature limit. The cooling protocol consisted of induced hypothermia for 60 min followed by 15 min of rewarming. Data for each parameter were collected every 5 min.
Monitoring
The brain temperature was monitored bilaterally using parenchymal catheters (Neurovent-PT and PT-O; Raumedic). Systemic temperatures were assessed with three thermometers; one in the rectum (AT-02005-0; Dixtal Biomédica, São Paulo, SP, Brasil), a second in the esophagus (AT-02005-0; Dixtal Biomédica), and a third at the pulmonary artery (93A-131H-7F; Baxter Edwards Critical Care, Irvine, CA, USA). All data were transmitted and stored on a multiparametric monitor (DX 2020; Philips/Dixtal).
Heart rate (HR), blood pressure, and cardiac output (CO) were also assessed. HR was monitored using three electrodes placed on the chest area (3 M Red Dot ECG Electrodes, St. Paul, MN, USA), mean arterial pressure (MAP) was determined using invasive arterial catheters positioned in the femoral artery (ASK-04018-HCA; Arrow International, Reading, PA, USA), and CO was measured with a pulmonary artery catheter (7F93A131H; Baxter Edwards Critical Care) using the intermittent thermodilution technique [43,44,45,46,47]. Data were captured and stored on a multiparametric monitor (DX 2020; Philips/Dixtal).
ICP data were collected along with right hemisphere temperature data using the same catheter (Neurovent-PT, Raumedic). Data were transmitted and shown on a multiparameter monitor (DX 2020; Philips/Dixtal).
Cerebral blood flow velocity (CBFv) was assessed using a transcranial Doppler (TCD) ultrasound system coupled to a 4–8 MHz multi-frequency transducer (MicroMaxx SonoSite; Bothell, WA, USA). It was necessary to perform a small craniotomy in the temporal bone to enable contact of the ultrasound transducer with dura mater for a better signal. TCD data were collected in all animals from the middle cerebral artery by a physician with expertise in this procedure (co-author EB Shu). Mean velocity, peak systolic velocity (SV), end-diastolic velocity (EDV), and resistance index (RI) were recorded. Through the same cranial window, ultrasound B Mode was used to obtain images of the intracranial space and confirm the position of the ICP catheter.
The PbtO2 in brain tissue was determined with the same catheter (Neurovent-PT-O; Raumedic) used for monitoring ICP, left hemisphere temperature, and PbtO2 simultaneously. These data were stored on a specific monitor (MPR2 logo; Raumedic).
Nasal, pharyngeal, and oral inspections were performed with direct laryngoscopy immediately after removal of the nasopharyngeal catheter. Possible lesions were described according to their severity, extent, and depth.
At the end of experimentation, all animals were euthanized with an additional dose of 1 g thiopental sodium and a rapid intravenous administration of 2 mEq/L/kg body weight of 19.1% KCl.
Statistical Methods
The recommended sample size was calculated by a statistician. A total of 10 animals were necessary to detect a 1 °C decrease in temperature from baseline with a power of 80% and significance level of 0.05 in a one-tailed paired test.
The investigation hypotheses of this study were as follows. H0: temperature reduction < 1 °C versus H1: temperature reduction ≥ 1 °C.
The Shapiro–Wilk test was used to assess whether physiological parameters and vital signs were normally distributed. Statistical comparisons between different time points were performed using either a paired t test for normally distributed variables or the Wilcoxon matched-pairs signed rank test non-normally distributed variables. The within-subject variance was assumed to be constant, and observations within subjects were independent. Fractional polynomial regression was used to fit models to predict cerebral temperature given the total time of nasopharyngeal cooling. Data are expressed as means ± standard deviations. Analyses were conducted with SPSS 17.0.0 (SPSS, Inc., Chicago, IL, USA).
Results
After applying the inclusion and exclusion criteria, a total of 10 healthy crossbred pigs were enrolled in a single center over a period of 8 weeks. The animals had a mean weight of 20.6 ± 1.8 kg.
Nasopharyngeal catheters were inserted in the left nostril and properly positioned at the nasopharyngeal cavity of all study animals. Water at a mean temperature of 0.60 ± 0.19 °C and mean flow rate of 2.20 ± 0.47 L/min was circulated inside the catheter during the study. The cooling intervention lasted 60 min, and the active rewarming occurred over a period of 15 min after the cooling intervention was stopped. Mean room temperature during the study protocol was 21.4 ± 1.15 °C.
At baseline, the mean left and right hemisphere temperatures were 38.82 ± 1.12 °C and 38.78 ± 1.16 °C, respectively, with no significant difference between hemispheres (p = 0.70). A significant temperature reduction in both hemispheres (left hemisphere temperature difference: − 1.47, p < 0.001; right hemisphere difference: − 0.99, p < 0.001) were noted within the first 5 min (Table 1; Fig. 3). The temperature gradient between the left and right hemispheres became significant after 20 min of cooling (left hemisphere temperature: 35.24 ± 1.49 °C; right hemisphere temperature: 36.06 ± 0.98 °C, p = 0.01). This difference remained stable until the end of the experiment.
Left (a) and right (b) hemisphere brain temperature during the first 60 min of the experiment in 10 pigs. Individual values are indicated by red dots and the mean value by the blue line. The following equations describe the temperature of the left and right hemispheres over time: \( {\text{Left}}\,{\text{brain}}\,{\text{temperature}} = 33.753 - 0.953 \times \left( {\frac{{{\text{time}} + 5}}{10}} \right)^{ - 2} + 4.426 \times \left( {\frac{{{\text{time}} + 5}}{10}} \right)^{ - 1} \)\( {\text{Right}}\,{\text{brain}}\,{\text{temperature}} = 34.815{-\!\!-}0.857 \times \left( {\frac{{{\text{time}} + 5}}{10}} \right)^{ - 2} + 3.727 \times \left( {\frac{{{\text{time}} + 5}}{10}} \right)^{ - 1} \) (Color figure online)
Comparison of systemic baseline temperatures measured at three different locations (esophagus, rectum, and pulmonary artery) revealed no significant differences (Table 2). During the intervention, there was no significant deviation from systemic baseline temperatures. After 60 min of intervention, the maximal reduction in rectal temperature was 0.23 ± 1.16 °C (p = 0.55) compared to baseline.
HR, MAP, and CO remained stable throughout the experiment (Table 3) compared to baseline values. Mean baseline HR was 117.00 ± 12.98 bpm, MAP was 76.90 ± 7.98 mmHg, and CO was 3.74 ± 0.62 L/min. ICP values did not change during the experiment (Table 4) compared to mean baseline ICP value of 9.00 ± 6.32 mmHg. According to the data collected using TCD ultrasound, SV, EDV, and RI mean values remained stable throughout the experiment (Table 5) compared to baseline values. Mean baseline values of SV, EDV, and RI were 40.86 ± 13.52 cm/s, 21.99 ± 5.16 cm/s, and 0.44 ± 0.08, respectively.
In brain tissue, the PbtO2 displayed significant variations compared to the baseline value of 54.53 ± 19.62 mmHg. PbtO2 was significantly reduced within the first 10 min and remained significantly decreased until the end of the cooling phase (Table 6). The curve of the PbtO2 reduction was proportional to that of brain temperature reduction. During the rewarming phase, values tended to return to baseline following the increase in brain temperatures. No mucosal lesions were detected during nasal, pharyngeal, or oral inspections after removing the nasopharyngeal catheter. Since there were no lesions, it was not possible to classify their severity, extent, or depth.
Discussion
This study demonstrated that a novel nasopharyngeal catheter was able to cool the brain of pigs by at least 1 °C in all animals with no local or systemic device-related adverse events. On average, the brain temperature was reduced by 4.5 °C after 60 min of cooling with no variation in core temperature. Systemic temperature parameters were not affected due to simultaneous counter-warming along with nasopharyngeal cooling.
Previous nasopharyngeal cooling methods failed to preserve core temperature. A relevant mean decrease in core temperature of up to 2.5 °C was observed [27] in animals and did not prevent systemic or cardiovascular complications [48]. Preliminary human studies with a gas-based method reported local adverse events [30]. Our study demonstrated that a water-based method was effective and safe while local and systemic adverse events were prevented during this experiment. Brain temperature was significantly decreased in both hemispheres which may be desired in global neurological lesions such as global ischemia following cardiac arrest. Nonetheless, brain temperature was preferentially reduced in the left side because catheters were only introduced in the left nostril. This effect may facilitate the decision to prioritize one side to be cooled in localized neurological conditions like stroke. Counter-warming with blankets and thermal mattresses was applied with the intention to prevent a core temperature reduction while cooling the nasopharyngeal surface. For this study, we considered the normal core body temperature of pigs as 38–40 °C [49, 50]. Whole-body cooling is the current method for therapeutic cooling and target temperature management [18, 21, 51] and plays an important role in fever control. Selective methods, such as the method described in the present study, may change this trend in the near future due to their effectiveness and favorable short-term safety profile.
Hypothermia (32–34 °C) causes hemodynamic disturbances in cardiac contractility, HR, and rhythm [52], which impose a relevant risk to treatment that may limit its benefits. Systemic hemodynamic parameters remained stable during our experiment. This finding suggests that cardiac complications possibly result from systemic cooling and that preferential brain cooling could prevent hemodynamic disturbances.
The influence of cooling on cerebral hemodynamics such as CBFv have been investigated using TCD ultrasound, and CBFv values are reportedly reduced in response to systemic cooling [16, 53, 54]. The results from previous studies can be justified by systemic hemodynamic variations secondary to whole-body cooling [52]. Our study was the first to achieve exclusive brain cooling which allowed us to determine the actual influence of localized cooling on CBFv in pigs. Our data demonstrated that there were no significant variations in cerebral hemodynamics during nasopharyngeal exclusive brain cooling which suggests an improved safety profile of our technique compared to that of whole-body cooling.
The PbtO2 in brain tissue was assessed in our study with the intention to determine the relationship between CBFv and oxygen demand of brain tissue under exclusive brain cooling. During the cooling phase, there was a significant decrease in PbtO2, which returned to baseline levels during the rewarming phase. The changes in PbtO2 were proportional to those in brain temperature. Since PbtO2 probes measure oxygen levels at the cellular level, this result was expected in healthy individuals because the coupling between oxygen demand and delivery remains preserved, and the oxygen demand of brain cells is physiologically reduced under lower temperatures. A leftward shift of the oxyhemoglobin dissociation curve [55, 56] during temperature reduction may also explain the decreased oxygen levels observed in this situation. In both cases, oxygen delivery is preserved and prevents harm. It is important to mention that we used healthy pigs in our study where autoregulation and physiological functions are preserved. In experimental or clinical studies with brain lesions, this pattern may be affected by loss of autoregulation, and comparisons to our data should be made with caution. The high levels of oxygen in brain tissue at baseline are defined by 100% FiO2 level. We decided to maintain this FiO2 level during the experiment to avoid any influence of its variations on PbtO2.
Preliminary human studies with nasopharyngeal cooling methods have reported adverse events such as nasal discoloration (11%), epistaxis (2%), and cold-induced tissue injury (1%) [30, 36]. In our study, no local lesions were detected during nasal, pharyngeal, or oral inspections after the experiment. We conjecture that our method did not induce local complications due to the temperature range of the operation (above 0 °C) which prevented cold-induced lesions in the mucosa caused by temperatures below this level. Local adverse events reported by previous studies are possibly related to the very low temperature of operation, below 0 °C [36].
The technique implemented in our study provided a substantial temperature reduction in the brain using a novel nasopharyngeal technique. The favorable results suggest that our method could be safer than whole-body and comparable to other selective brain cooling methods based on short-term analysis, but further studies are required for comparison of techniques. The small size of the catheter when connected to a portable water-cooling unit allows the implementation of therapeutic hypothermia or temperature control in pre-hospital or military settings due to its portability. The short-term time window validated in our study may also be desired for preventive brain cooling during cardiac surgery. Clinical studies are warranted to determine the relevance of our results and safety of our technique in humans.
Limitations
The experiment was conducted in a healthy small porcine model over a short period of time and with no control group. Pigs have anatomical and physiological similarities to humans and are an experimental model widely used in the field of therapeutic hypothermia [57, 58]. The average weight of an adult human is 62 kg with a brain weight of 1350 g [59], while the average body weight of the 2-month-old Landrace pigs used in our study was 20.6 kg with an average brain weight of 75 g [60]. In our experiment, the brain mass of the pigs was 18 times smaller than the average human brain. Therefore, caution must be exercised when predicting cooling rates of our investigational catheter in humans. We hypothesize that a cooling rate 10-20 times smaller could be achieved in a human study.
The choice for healthy animals relied on the main objective of the study which was to determine whether the novel nasopharyngeal catheter could exclusively cool the brains of pigs. However, the small study size may have favored positive results. Despite the short intervention period, 60 min was sufficient to determine the short-term efficacy and safety of the method. Rapid onset pre-hospital cooling seems to be beneficial according to some authors [61, 62], while intra-arrest cooling may improve survival rates [63, 64]. Therefore, a short-period induction technique followed by a stabilized technique for maintenance could be desired in certain situations. Further studies are needed to determine if our technique is suitable for more prolonged periods.
We assumed that a control group was not essential since we considered the baseline values of each animal as the control for each comparison. The speed of brain cooling could have been influenced by ambient exposure, but the expressive reduction in brain temperatures during our experimental protocol was significant and was immediately elevated during the rewarming phase. Anesthesia did not affect brain cooling rate because it was delivered at a constant room temperature and low volume infusion rate throughout the experiment; however, it could have affected other outcome parameters. Caution should be exercised when deriving clinical implications from procedures conducted in non-human animals.
Conclusions
In this study, a novel nasopharyngeal cooling catheter was able to effectively induce and maintain exclusive brain cooling when combined with effective counter-warming means. The method is based on a catheter with a semi-expandable section which allows extensive contact with the rugged surface of the nasal, oral, and nasopharyngeal surface. The expansion occurred upon exertion of pressure generated by the water pumped inside the catheter in its closed loop arrangement. Exclusive brain cooling was shown to be safe with no device-related local or systemic complications and may be desired in selected patient populations.
References
Stroke. World Heart Federation [cited 2016 Jun 26]. http://www.world-heart-federation.org/cardiovascular-health/stroke/.
Cheng P, Yin P, Ning P, et al. Trends in traumatic brain injury mortality in China, 2006–2013: a population-based longitudinal study. PLoS Med. 2017;14:e1002332.
Majdan M, Plancikova D, Brazinova A, et al. Epidemiology of traumatic brain injuries in Europe: a cross-sectional analysis. Lancet Public Health. 2016;1:e76–83.
Majdan M, Plancikova D, Maas A, et al. Years of life lost due to traumatic brain injury in Europe: a cross-sectional analysis of 16 countries. PLoS Med. 2017;14:e1002331.
Macdonald R. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2013;10:44–58.
Rowland M, Hadjupavlou G, Kelly M, Westbrook J, Pattinson K. Delayed cerebral ischaemia after subarachnoid haemorrhage: looking beyond vasospasm. Br J Anaesth. 2012;109:315–29.
Polderman K. Application of therapeutic hypothermia in the intensive care unit. Intensive Care Med. 2004;30:757–69.
Cobas MA, Vera-Arroyo A. Hypothermia. Adv Anesth. 2017;35:25–45.
The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56.
Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63.
Rabinstein A. How cool it is: targeted temperature management for brain protection post-cardiac arrest. Semin Respir Crit Care Med. 2016;37:34–41.
Small DL, Morley P, Buchan AM. Biology of ischemic cerebral cell death. Prog Cardiovasc Dis. 1999;42:185–207.
Schmitt KRL, Tong G, Berger F. Mechanisms of hypothermia-induced cell protection in the brain. Mol Cell Pediatr. 2014; 1 [cited 2019 Jan 11]. http://www.molcellped.com/content/1/1/7.
Liu X, Wu D, Wen S, et al. Mild therapeutic hypothermia protects against cerebral ischemia/reperfusion injury by inhibiting miR-15b expression in rats. Brain Circ. 2017;3:219.
Carpenter KLH, Czosnyka M, Jalloh I, et al. Systemic, local, and imaging biomarkers of brain injury: more needed, and better use of those already established? Front Neurol. 2015; 6 [cited 2019 Jan 11]. http://journal.frontiersin.org/Article/10.3389/fneur.2015.00026/abstract.
Mrozek S, Vardon F, Geeraerts T. Brain temperature: physiology and pathophysiology after brain injury. Anesthesiol Res Pract. 2012;2012:1–13.
Wang H, Wang B, Normoyle KP, et al. Brain temperature and its fundamental properties: a review for clinical neuroscientists. Front Neurosci. 2014; 8 [cited 2019 Jan 11]. http://journal.frontiersin.org/article/10.3389/fnins.2014.00307/abstract.
Callaway CW, Donnino MW, Fink EL, et al. Part 8: post-cardiac arrest care 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S465–82.
Neumar RW, Bhanji F, Brooks SC, et al. American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:293.
Hazinski MF, Nolan JP, Aickin R, et al. Part 1: executive summary: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2015;132:S2–39.
Arrich J, Holzer M, Havel C, Müllner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Anaesthesia, Critical and Emergency Care Group, editor. Cochrane Database Syst Rev. 2016 [cited 2018 Feb 21]. http://doi.wiley.com/10.1002/14651858.CD004128.pub4.
Crompton EM, Lubomirova I, Cotlarciuc I, Han TS, Sharma SD, Sharma P. Meta-analysis of therapeutic hypothermia for traumatic brain injury in adult and pediatric patients. Crit Care Med. 2017;45:575–83.
Fox JL, Vu EN, Doyle-Waters M, Brubacher JR, Abu-Laban R, Hu Z. Prophylactic hypothermia for traumatic brain injury: a quantitative systematic review. CJEM. 2010;12:355–64.
Choi JH, Pile-Spellman J. Selective brain hypothermia. Handb Clin Neurol. 2018 [cited 2019 Jan 10]; 157: 839–852. https://linkinghub.elsevier.com/retrieve/pii/B9780444640741000525.
Dumitrascu OM, Lamb J, Lyden PD. Still cooling after all these years: meta-analysis of pre-clinical trials of therapeutic hypothermia for acute ischemic stroke. J Cereb Blood Flow Metab. 2016;36:1157–64.
Patel RAG, McMullen PW. Neuroprotection in the treatment of acute ischemic stroke. Prog Cardiovasc Dis. 2017;59:542–8.
Boller M, Lampe JW, Katz JM, Barbut D, Becker LB. Feasibility of intra-arrest hypothermia induction: a novel nasopharyngeal approach achieves preferential brain cooling. Resuscitation. 2010;81:1025–30.
Cavaciu L, Allers M, Enblad P, Lunderquist A, Wieloch T, Rubertsson S. Intranasal selective brain cooling in pigs. Resuscitation. 2008;76:83–8.
Abou-Chebl A, Sung G, Barbut D, Torbey M. Local brain temperature reduction through intranasal cooling with the rhinochill device: preliminary safety data in brain-injured patients. Stroke. 2011;42:2164–9.
Poli S, Purrucker J, Priglinger M, Sykora M, Diedler J, Rupp A, et al. Safety evaluation of nasopharyngeal cooling (RhinoChill®) in stroke patients: an observational study. Neurocrit Care. 2014;20:98–105.
Einer-Jensen N, Khorooshi MH, Petersen MB, Svendsen P. Rapid brain cooling in intubated pigs through nasal flushing with oxygen: prevention of brain hyperthermia. Acta Vet Scand. 2001;42:6.
Fazel Bakhsheshi M, Wang Y, Keenliside L, Lee T-Y. A new approach to selective brain cooling by a Ranque-Hilsch vortex tube. Intensive Care Med Exp. 2016; 4 [cited 2016 Oct 28]. http://icm-experimental.springeropen.com/articles/10.1186/s40635-016-0102-5.
Chava R, Zviman M, Raghavan MS, et al. Rapid induction of therapeutic hypothermia using transnasal high flow dry air. Ther Hypothermia Temp Manag. 2017;7:50–6.
Sedlacik J, Kjørstad Å, Nagy Z, et al. Feasibility study of a novel high-flow cold air cooling protocol of the porcine brain using MRI temperature mapping. Ther Hypothermia Temp Manag. 2018;8:45–52.
Westermaier T, Nickl R, Koehler S, et al. Selective brain cooling after traumatic brain injury: effects of three different cooling methods—case report. J Neurol Surg A Cent Eur Neurosurg. 2017;78:397–402.
Busch H-J, Eichwede F, Födisch M, et al. Safety and feasibility of nasopharyngeal evaporative cooling in the emergency department setting in survivors of cardiac arrest. Resuscitation. 2010;81:943–9.
Castrén M, Nordberg P, Svensson L, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation. 2010;122:729–36.
Council NR, Studies DEL, Research ILA, Research CSHIURSDC. Scientific and humane issues in the use of random source dogs and cats in research. National Academies Press; 2009. https://books.google.com.br/books?id=pt76G2W32l0C.
American Physiological Society. Guiding Principles for the care and use of vertebrate animals in research and training. 2019 [cited 2019 Jan 16]. http://www.the-aps.org/mm/SciencePolicy/About/Policy-Statements/Guiding-Principles.html.
Concea. [cited 2019 Mar 12]. http://www.mctic.gov.br/mctic/opencms/institucional/concea/paginas/concea.html.
Andersen ML, de Angelis K, de Souza RL, et al. Conselho nacional de controle de experimentação animal [National Animal Experimentation Control Council] Sep 26, 2016 p. 387.
Flecknell PA. Laboratory animal anaesthesia: a practical introduction for research workers and technicians. 2nd ed. Amsterdam: Elsevier Acad. Press; 2005.
Munro HM, Wood CE, Taylor BL, Smith GB. Continuous invasive cardiac output monitoring–the Baxter/Edwards Critical-Care Swan Ganz IntelliCath and Vigilance system. Clin Intensive Care. 1994;5:52–5.
Yamada T, Tsutsui M, Sugo Y, et al. Multicenter study verifying a method of noninvasive continuous cardiac output measurement using pulse wave transit time: a comparison with intermittent bolus thermodilution cardiac output. Anesth Analg. 2012;115:82–7.
Hofer CK, Cannesson M. Monitoring fluid responsiveness. Acta Anaesthesiol Taiwan. 2011;49:59–65.
Lanspa MJ, Grissom CK, Hirshberg EL, Jones JP, Brown SM. Applying dynamic parameters to predict hemodynamic response to volume expansion in spontaneously breathing patients with septic shock. Shock. 2013;39:462.
Muller L, Bobbia X, Toumi M, et al. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: need for a cautious use. Crit Care. 2012;16:R188.
Wolfson MR, Malone DJ, Wu J, et al. Intranasal perfluorochemical spray for preferential brain cooling in sheep. Neurocrit Care. 2008;8:437–47.
Musk GC, Costa RS, Tuke J. Body temperature measurements in pigs during general anaesthesia. Lab Anim. 2016;50:119–24.
Reece WO, Erickson HH, Goff JP, Uemura EE, editors. Body temperature and its regulation. Dukes’ physiology of domestic animals, vol. 150. 13th ed. Ames: Wiley; 2015.
Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197–206.
Soleimanpour H, Rahmani F, EJ Golzari S, Safari S. Main complications of mild induced hypothermia after cardiac arrest: a review article. J Cardiovasc Thorac Res. 2014 [cited 2018 Oct 19]. http://journals.tbzmed.ac.ir/JCVTR/Abstract/JCVTR_1485_20140210224645.
Keller E, Steiner T, Fandino J, Schwab S, Hacke W. Changes in cerebral blood flow and oxygen metabolism during moderate hypothermia in patients with severe middle cerebral artery infarction. Neurosurg Focus. 2000;8:e4.
Flynn LMC, Rhodes J, Andrews PJD. Therapeutic hypothermia reduces intracranial pressure and partial brain oxygen tension in patients with severe traumatic brain injury: preliminary data from the Eurotherm3235 trial. Ther Hypothermia Temp Manag. 2015;5:143–51.
Bacher A. Effects of body temperature on blood gases. Intensive Care Med. 2004;31:24–7.
Zanelli S, Buck M, Fairchild K. Physiologic and pharmacologic considerations for hypothermia therapy in neonates. J Perinatol. 2011;31:377–86.
Wang H, Barbut D, Tsai M-S, Sun S, Weil MH, Tang W. Intra-arrest selective brain cooling improves success of resuscitation in a porcine model of prolonged cardiac arrest. Resuscitation. 2010;81:617–21.
Cho JH, Ristagno G, Li Y, Sun S, Weil MH, Tang W. Early selective trans-nasal cooling during CPR improves success of resuscitation in a porcine model of prolonged pulseless electrical activity cardiac arrest. Resuscitation. 2011;82:1071–5.
Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–55.
de Andrade AF, Soares MS, Patriota GC, et al. Experimental model of intracranial hypertension with continuous multiparametric monitoring in swine. Arq Neuropsiquiatr. 2013;71:802–6.
Lee BK, Jeung KW, Jung YH, et al. Relationship between timing of cooling and outcomes in adult comatose cardiac arrest patients treated with targeted temperature management. Resuscitation. 2017;113:135–41.
Arrich J, Holzer M, Havel C, Müllner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2016 [cited 2018 Feb 21]. http://doi.wiley.com/10.1002/14651858.CD004128.pub4.
Garrett JS, Studnek JR, Blackwell T, et al. The association between intra-arrest therapeutic hypothermia and return of spontaneous circulation among individuals experiencing out of hospital cardiac arrest. Resuscitation. 2011;82:21–5.
Scolletta S, Taccone F, Nordberg P, Donadello K, Vincent J-L, Castren M. Intra-arrest hypothermia during cardiac arrest: a systematic review. Crit Care. 2012;16:R41.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Protocol/project development was performed by BLP, EBSS, ES, IB, MO, RF, AC, MT; data collection or management was done by BLP, EBSS, IB, MO; data analysis was performed by BLP, EBSS, ES, MO, RF, AC, MT; manuscript writing/editing other was done by BLP, EBSS, ES, MO, RF, AC, MT.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that Bernardo Lembo Conde de Paiva and Raphael Einsfeld Simões Ferreira have the Intellectual property rights of the novel catheter derived from this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Paiva, B.L.C., Bor-Seng-Shu, E., Silva, E. et al. Inducing Brain Cooling Without Core Temperature Reduction in Pigs Using a Novel Nasopharyngeal Method: An Effectiveness and Safety Study. Neurocrit Care 32, 564–574 (2020). https://doi.org/10.1007/s12028-019-00789-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-019-00789-2