Abstract
Background
Conivaptan is an arginine-vasopressin-receptor antagonist approved for the treatment of hyponatremia. We hypothesized that administration of conivaptan to normonatremic patients with traumatic brain injury (TBI) is safe and could reduce intracranial pressure (ICP).
Methods
Open-label, randomized, controlled trial enrolling 10 subjects within 24 h of severe TBI to receive a single 20 mg dose of conivaptan (n = 5) or usual care (n = 5). The primary endpoint was the evaluation of the safety profile defined by serum sodium increases averaging >1 mEq/h when measured every 4 h and any adverse events. Secondary endpoints were 48-h serum sodium, sodium load, change in ICP, and urine output.
Results
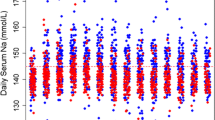
Ten patients were included in the intention-to-treat analysis. Three patients (2 conivaptan, 1 usual care group) experienced brief sodium increases averaging >1 mEq/h, with no patients achieving Na >160 mEq/l. There were no drug-related serious adverse events. At 48 h, the mean sodium was 142 ± 6 mEq/l (conivaptan) and 144 ± 10 mEq/l (usual care, P = 0.71). 48-h sodium load was 819 ± 724 mEq in the conivaptan and 1,137 ± 1,165 mEq in the usual care group (P = 0.62). At 4 h, serum sodium was higher (P = 0.02) and ICP was lower (P = 0.046) in the conivaptan compared with usual care group. 24-h but not 48-h urine output was different between the two groups (P < 0.01 and P = 0.20, respectively).
Conclusions
These data suggest that a single dose conivaptan is safe in non-hyponatremic patients with severe TBI and may reduce ICP. Further studies are needed to establish the effect of conivaptan on clinically relevant endpoints, and its role in the management of intracranial hypertension.



Similar content being viewed by others
References
Rangel-Castilla L, Gopinath S, Robertson CS. Management of intracranial hypertension. Neurol Clin. 2008;26:521–41.
Wright WL, Asbury WH, Gilmore JL, Samuels OB. Conivaptan for hyponatremia in the neurocritical care unit. Neurocrit Care. 2009;11:6–13.
Dhar R, Murphy-Human T. A bolus of conivaptan lowers intracranial pressure in a patient with hyponatremia after traumatic brain injury. Neurocrit Care. 2011;14(1):97–102.
Murphy T, Dhar R, Diringer M. Conivaptan bolus dosing for the correction of hyponatremia in the neurointensive care unit. Neurocrit Care. 2009;11:14–9.
Naidech AM, Paparello J, Leibling SM, et al. Use of conivaptan (Vaprisol) for hyponatremic neuro-ICU patients. Neurocrit Care. 2010;13:57–61.
Liu X, Nakayama S, Amiry-Moghaddam M, Ottersen OP, Bhardwaj A. Arginine-vasopressin V1 but not V2 receptor antagonism modulates infarct volume, brain water content, and aquaporin-4 expression following experimental stroke. Neurocrit Care. 2010;12:124–31.
Trabold R, Krieg S, Scholler K, Plesnila N. Role of vasopressin V(1a) and V2 receptors for the development of secondary brain damage after traumatic brain injury in mice. J Neurotrauma. 2008;25:1459–65.
Taya K, Gulsen S, Okuno K, Prieto R, Marmarou CR, Marmarou A. Modulation of AQP4 expression by the selective V1a receptor antagonist, SR49059, decreases trauma-induced brain edema. Acta Neurochir. 2008;102:425–9.
Kozniewska E, Romaniuk K. Vasopressin in vascular regulation and water homeostasis in the brain. J Physiol Pharmacol. 2008;59(Suppl 8):109–16.
Bemana I, Nagao S. Treatment of brain edema with a nonpeptide arginine vasopressin V1 receptor antagonist OPC-21268 in rats. Neurosurgery. 1999;44:148–54. (discussion 54–5).
Kagawa M, Nagao S, Bemana I. Arginine vasopressin receptor antagonists for treatment of vasogenic brain edema: an experimental study. J Neurotrauma. 1996;13:273–9.
Fernandez N, Martinez MA, Garcia-Villalon AL, Monge L, Dieguez G. Cerebral vasoconstriction produced by vasopressin in conscious goats: role of vasopressin V(1) and V(2) receptors and nitric oxide. Br J Pharmacol. 2001;132:1837–44.
Suzuki Y, Satoh S, Kimura M, et al. Effects of vasopressin and oxytocin on canine cerebral circulation in vivo. J Neurosurg. 1992;77:424–31.
Suzuki Y, Satoh S, Oyama H, Takayasu M, Shibuya M, Sugita K. Vasopressin mediated vasodilation of cerebral arteries. J Auton Nerv Syst. 1994;49(Suppl):S129–32.
Tsugane S, Suzuki Y, Kano T, Takayasu M, Shibuya M, Sugita K. Differing effects of vasopressin on regional cerebral blood flow of dogs following intracisternal vs. intra-arterial administration. Life Sci. 1994;54:PL241–6.
Lluch S, Conde MV, Dieguez G, et al. Evidence for the direct effect of vasopressin on human and goat cerebral arteries. J Pharmacol Exp Ther. 1984;228:749–55.
Adler S, Verbalis JG, Williams D. Effect of rapid correction of hyponatremia on the blood-brain barrier of rats. Brain Res. 1995;679:135–43.
Acknowledgment
Funding: Financial support for this investigator initiated study was provided by Astellas. The sponsor had no role in the design, interpretation, and reporting of the study findings.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galton, C., Deem, S., Yanez, N.D. et al. Open-Label Randomized Trial of the Safety and Efficacy of a Single Dose Conivaptan to Raise Serum Sodium in Patients with Traumatic Brain Injury. Neurocrit Care 14, 354–360 (2011). https://doi.org/10.1007/s12028-011-9525-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-011-9525-8