Abstract
Acute traumatic brain injury (TBI) is a major cause of mortality and disability worldwide. Intracranial pressure (ICP)-lowering is a critical management priority in patients with moderate to severe acute TBI. We aimed to evaluate the clinical efficacy and safety of hypertonic saline (HTS) versus other ICP-lowering agents in patients with TBI. We conducted a systematic search from 2000 onward for randomized controlled trials (RCTs) comparing HTS vs. other ICP-lowering agents in patients with TBI of all ages. The primary outcome was the Glasgow Outcome Scale (GOS) score at 6 months (PROSPERO CRD42022324370). Ten RCTs (760 patients) were included. Six RCTs were included in the quantitative analysis. There was no evidence of an effect of HTS on the GOS score (favorable vs. unfavorable) compared with other agents (risk ratio [RR] 0.82, 95% confidence interval [CI] 0.48–1.40; n = 406; 2 RCTs). There was no evidence of an effect of HTS on all-cause mortality (RR 0.96, 95% CI 0.60–1.55; n = 486; 5 RCTs) or total length of stay (RR 2.36, 95% CI − 0.53 to 5.25; n = 89; 3 RCTs). HTS was associated with adverse hypernatremia compared with other agents (RR 2.13, 95% CI 1.09–4.17; n = 386; 2 RCTs). The point estimate favored a reduction in uncontrolled ICP with HTS, but this was not statistically significant (RR 0.52, 95% CI 0.26–1.04; n = 423; 3 RCTs). Most included RCTs were at unclear or high risk of bias because of lack of blinding, incomplete outcome data, and selective reporting. We found no evidence of an effect of HTS on clinically important outcomes and that HTS is associated with adverse hypernatremia. The included evidence was of low to very low certainty, but ongoing RCTs may help to the reduce this uncertainty. In addition, heterogeneity in GOS score reporting reflects the need for a standardized TBI core outcome set.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute traumatic brain injury (TBI) is a major cause of mortality and disability worldwide [1]. In the United Kingdom, TBI is the most common cause of death in patients under 40 years of age [2]. Raised intracranial pressure (ICP) secondary to TBI increases the risk of brain herniation and is associated with poorer clinical outcomes [3]. Thus, lowering ICP is a critical management priority in patients with moderate to severe acute TBI.
Hyperosmolar therapies, such as hypertonic saline (HTS) and mannitol, are in routine clinical use for lowering ICP in TBI. Historically, both agents were thought to produce an ICP-lowering effect by drawing interstitial fluid within edematous brain tissue intravascularly. More recently, their mechanism of action is increasingly understood to involve complex alterations in blood viscosity and microcirculatory changes resulting in pial arteriolar constriction, decreased cerebral blood volume, and reduced ICP [4, 5]. Despite increasing popularity of HTS in this setting and positive results from previous studies suggesting potential clinical benefits, the most recent Brain Trauma Foundation guidelines (2016) state that there was “insufficient evidence available from comparative studies to support a formal recommendation” for its use [6,7,8]. Severe hypernatremia has been noted as a potential adverse effect associated with HTS use [9]. Moreover, a recent Cochrane review concluded that there was weak evidence to suggest HTS has no effect on long-term neurological outcome compared with mannitol, although this review was released prior to publication of the largest randomized trial investigating HTS infusion in patients with acute TBI (the continous hyperosmolar therapy for traumatic brain-injured patients (COBI) trial) [10, 11]. The COBI trial included 370 adults with moderate to severe TBI and found no evidence of an effect of a continuous HTS infusion compared with standard care on long-term neurological function.
Therefore, it remains unclear whether HTS offers any clinical benefit over other ICP-lowering methods in terms of long-term functional outcome, all-cause mortality, ICP control, and adverse effects. This review seeks a definitive answer to this question to guide clinical practice and inform future research.
Methods
This report was prepared according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline [12]. Our review protocol was prospectively registered on PROSPERO (CRD4202234370).
Eligibility Criteria
We included randomized controlled trials (RCTs) comparing the effect of HTS bolus(es) or infusion versus other ICP-lowering agents on clinical outcomes of interest in patients of all ages receiving critical care for acute TBI. Because HTS was licensed as a hyperosmolar agent for lowering ICP in 2004, studies were selected from 2000 onward to ensure that results are reflective of current clinical practice. Nonhuman studies, conference abstracts, and those published in languages other than English were excluded.
Our primary outcome was “favorable” Glasgow Outcome Scale (GOS) score at 6 months [13] (Fig. 1). A full description of GOS score criteria is provided in the Supplementary Material. Secondary outcomes were all-cause mortality, changes in ICP, proportion of patients with uncontrolled ICP, length of stay (hospital and/or intensive care unit [ICU]), and adverse events, including pulmonary edema and rebound phenomenon.
Search Strategy
MEDLINE, Cochrane CENTRAL (Cochrane Central Register of Controlled Trials), Embase, ISI (Institute for Scientific Information) Web of Science, Scopus, and clinical trial registries (ClinicalTrials.gov, World Health Organization International Trials Registry, Chinese Clinical Trials Registry) were initially searched on April 10, 2022, according to a predefined search strategy for each database. This search was repeated on November 19, 2022. The search strategies were developed in collaboration with an experienced librarian from Bodleian Libraries, University of Oxford. Reference lists of identified trials were searched for further relevant literature, and individual study authors were contacted to request additional data if necessary. Key search terms included “hypertonic saline,” “traumatic brain injury,” and “intracranial pressure.” Individual search strategies can be found in the Supplemental Material.
Study Selection and Data Extraction
Titles, abstracts, and full texts of identified studies were screened in duplicate by two independent authors (KB, WM) against prespecified inclusion and exclusion criteria (see Eligibility criteria section). Any discrepancies in the screening process were discussed until consensus was reached, and in the event of a disagreement, a third author (AS) was assigned to adjudicate. Study authors were contacted if additional data were required for inclusion in the quantitative analysis. Data were extracted in duplicate by two independent authors using a pre-piloted spreadsheet.
Risk of Bias Assessment
The Cochrane Collaboration’s domain-based Risk of Bias 1 tool was used to assess risk of bias for each included study. Any discrepancies were discussed until consensus was reached. A third assessor was approached to adjudicate if consensus was not possible. Adjudication was only required on one occasion.
Data Synthesis
Data were entered into the Cochrane Collaboration’s systematic review software (RevMan 5, 2011) Heterogeneity between studies was assessed with the use of I2 [14]. Data were synthesized to obtain pooled estimates of relative risks (95% confidence interval [CI]) or mean difference (95% CI) as appropriate using a random-effects model for primary and secondary outcomes. Owing to variations in reporting of GOS scores between studies, the primary outcome (GOS score at 6 months) was dichotomized into “favorable” or “unfavorable” functional outcome (Fig. 1). This review outcome was reported as a pooled risk ratio (RR) with a corresponding 95% CI. Forest plots were produced for each outcome of interest. Where possible, continuous variables were reported as weighted mean or standardized mean difference as appropriate.
Where data could not be pooled, narrative syntheses were performed. Subgroup analyses focusing on administration factors, age group, and TBI severity were prespecified to determine whether these factors affect outcomes of interest. Moreover, a sensitivity analysis was planned to investigate the influence of high risk of bias studies. However, few studies were identified for inclusion, and the majority of these consisted of small sample sizes. This precluded our ability to perform further meaningful subgroup or sensitivity analyses using currently available data.
Certainty of Evidence
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to assess the overall certainty of the evidence [15].
Results
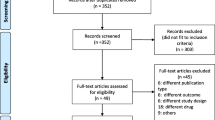
Of 65 studies identified, 13 underwent full-text screening after title and abstract screening (Fig. 2). Three studies were excluded after full-text screening because of incorrect study design. Of the ten remaining studies, six were included in the meta-analysis and three were included in narrative syntheses. One ongoing multicenter RCT (Sugar or Salt) was identified (ISRCTN16075091).
Description of Included Studies
Details of the included trials are shown in Table 1. The ten trials comprised a total of 760 patients receiving critical care for brain injury in the countries France, India, Iran, Germany, Egypt, and Israel. There were only three multicenter trials. Two trials included patients with spontaneous intracerebral or subarachnoid hemorrhage in addition to patients with acute TBI. Because TBI subgroup data were unavailable for both trials, these were included in narrative syntheses and omitted from the meta-analysis.
The majority of trials were conducted in patients aged 18 years and older. One trial included pediatric patients only (1–16 years old), whereas two others included patients aged 15–70 years and 16 years and older, respectively. One additional trial included patients of all ages. Six two-arm trials compared varying concentrations of intravenous (IV) HTS boluses with IV mannitol boluses. Two trials had three arms: one compared HTS boluses versus continuous HTS infusion versus mannitol boluses, and the other compared HTS boluses with two different concentrations of mannitol. One trial compared continuous HTS infusion with HTS boluses. Mannitol was the key comparator in the eight remaining trials. Concentrations and method of administration (bolus versus continuous infusion) of hyperosmolar agents varied between studies and are summarized in Table 1. One ongoing clinical trial was identified.
Risk of Bias Assessment
The risk of bias assessment for individual trials is shown in Fig. 3. Nearly all trials were at high risk for lack of blinding of participants and personnel because of a presumed inability to blind interventions in the critical care setting. Two trials rated low risk for this domain prohibited any additional therapeutic intervention (for example, nursing, manipulation of ventilatory variables, or vasoactive support) during the study period. Allocation concealment was rated as unclear risk for eight trials because of lack of clarity in study methods. Similarly, protocols were unavailable for most included trials, which resulted in a rating of unclear risk of reporting bias for six trials. One trial was considered low risk for every domain [24].
Effect of Interventions
GOS Score at 6 Months
Five trials reported a GOS score at 6 months. However, only two of these trials were suitable for meta-analysis. There was no evidence of an effect of HTS on favorable GOS score in patients with acute TBI and raised ICP (RR 0.82, 95% CI 0.48–1.40, P = 0.47, I2 = 45%, 2 RCTs, 406 participants) (Fig. 4). The remaining three trials were reported narratively (Table 2) and showed no difference in GOS score between treatment groups (P > 0.05, 3 RCTs, n = 80) [20, 21, 23].
All-Cause Mortality by 6 Months
There was no evidence of an effect of HTS on all-cause mortality by 6 months in patients with acute TBI (RR 0.96, 95% CI 0.60–1.55, P = 0.87, I2 = 41%, 5 RCTs, 486 participants) (Fig. 5). An additional trial comparing continuous 3% HTS infusion with intermittent 3% HTS boluses reported no difference in ICU mortality between the two groups, suggesting that the mode of HTS delivery had no impact on early mortality (P > 0.05, 50 participants) [24]. No trials reported reasons for deaths, but results from the COBI trial suggest that nearly all deaths occurred in both groups within the first 100 days from randomization [11].
Adverse Hypernatremia
There was variation in study authors’ definitions of adverse hypernatremia across the four trials included (Table 3). Two trials reported zero events in both the HTS and comparison groups [19, 20]. Therefore, only two studies were included in the meta-analysis, which showed that HTS use is associated with an increased risk of hypernatremia (RR 2.13, 95% CI 1.09–4.17, P = 0.03, I2 = 0%, 2 RCTs, 386 participants) (Fig. 6) [11, 16]. However, it should be noted that the multicenter COBI RCT comparing continuous infusion of a high concentration of HTS (20%) with other ICP-lowering agents accounted for the majority of the weighting for this point estimate [11]. Thus, it is possible that the reason for a higher risk of severe hypernatremia in the intervention group is largely due to the high concentration of HTS given continuously for at least 48 h, and these results should be interpreted within this context.
Uncontrolled ICP
Six trials reported “uncontrolled ICP” as an outcome, of which three were included in the meta-analysis [11, 20, 23]. Definitions of this outcome varied. One study defined this outcome as requirement of Brain Trauma Foundation guidelines “stage 3 therapies,” including barbiturates to lower ICP [11]. Vialet et al. [23] defined treatment failure as sustained raised ICP greater than 35 mm Hg despite two consecutive infusions of hyperosmolar therapy. Finally, Jagannatha et al. [20] defined this outcome as “persistently elevated ICP greater than 20 mmHg despite a maximum of three doses of hyperosmolar therapy,” necessitating the use of further ICP-lowering measures, including barbiturates, propofol, hyperventilation, cerebral spinal fluid drainage, or decompressive craniectomy. The meta-analysis showed no evidence of an effect of HTS on reducing ICP compared with other agents (RR 0.52, 95% CI 0.26–1.04, P = 0.07, I2 = 23%, 3 RCTs, 423 participants) (Fig. 7).
Three trials reported this outcome as follows: average time ICP exceeded 20 mm Hg, barbiturate requirement, and/or episodes of refractory ICP after three consecutive doses of hyperosmolar therapy [16, 21, 23]. Collectively, results from two of the trials showed no difference in the incidence of uncontrolled ICP between HTS and comparator groups (P > 0.05, 3 RCTs, 62 participants) [16, 21]. One trial showed that ICP exceeded 25 mm Hg for a shorter duration of time in the HTS group compared with the control group, although the authors did not provide baseline ICP data from participants at the start of the study period, which complicates interpretation of this result [23].
Length of stay (hospital or ICU)
The meta-analysis showed no evidence of an effect of HTS on total length of hospital stay compared with comparator agents (RR 2.36, 95% CI − 0.53 to 5.25, P = 0.11, I2 = 0%, 3 RCTs, 101 participants) (Fig. 8). Similarly, the meta-analysis showed no evidence of an effect of HTS on length of ICU stay (RR − 0.44, 95% CI − 2.85 to 1.97, P = 0.72, I2 = 0%, 3 RCTs, 101 participants) (Fig. 9). One additional trial reported no difference in length of ICU stay as a median and interquartile range (median 16 days in HTS group compared with 15 days in control group, difference = 1.0 day, 95% CI − 1.0 to 4.0 days, 370 participants) [11]. Additionally, Wahdan et al. [24] reported no difference in length of ICU stay when comparing continuous 3% HTS infusion with intermittent 3% HTS boluses (17.5 ± 11.8 and 17.2 ± 12.9, respectively, P = 0.36, 50 participants).
Reduction in ICP
Five trials reported ICP reduction as an outcome (605 participants), and these are described in Table 4 [11, 16, 20,21,22]. These trials could not be included in the meta-analysis because of variability of outcome reporting. Overall, there was no consistent effect of HTS on lowering ICP compared with other agents in patients with acute TBI.
Pulmonary Edema and Rebound Phenomenon
Although pulmonary edema and rebound phenomenon are potential complications of HTS use [16, 17], none of the included trials reported either as outcomes, with the exception of Francony et al. [17], who reported that there were no instances of rebound phenomenon during the study.
Certainty of Evidence
The certainty of evidence ranged from low to very low across all outcomes (Table 5). Common reasons for downgrading were imprecision, differences in estimated effect size, and suspected publication bias.
Discussion
Key Findings
Our systematic review identified ten RCTs enrolling 760 patients of all ages with acute TBI. The main findings were the following: (1) there was no evidence of an effect of HTS compared with other agents (mainly mannitol) on long-term neurological outcome in patients with raised ICP; (2) similarly, there was no evidence of a beneficial effect of HTS on all-cause mortality, uncontrolled ICP, length of hospital or ICU stay, and ICP reduction; and (3) HTS may be associated with increased risk of adverse hypernatremia. However, 95% CIs were wide for all studied outcomes. Thus, it is difficult to elucidate clinically meaningful differences between HTS and other ICP-lowering strategies, including mannitol.
Overall, our results challenge previous studies [6, 7] that suggest HTS is more effective than its comparators (e.g., mannitol) and are congruent with findings of a recent Cochrane review that showed there is weak evidence that HTS is no better than mannitol for long-term management of TBI [10]. Despite the finding that HTS is associated with adverse hypernatremia compared with other agents, this result should be interpreted with caution because one large multicenter trial accounts for the majority of the weighting for this point estimate [11]. Importantly, this trial investigated the continuous infusion (at least 48 h) of a higher concentration of HTS (20%) than is normally used clinically (range 1.8–5%). Thus, it is possible that prolonged continuous infusion of concentrated HTS is largely responsible for the apparent increased risk of hypernatremia in the patients studied. On the contrary, other studies have reported no difference in plasma sodium concentration when comparing patients receiving HTS versus those receiving mannitol boluses, which might suggest a failure to achieve a hyperosmolar state when certain administration techniques are used [17, 20]. The effect of bolus versus continuous infusion of HTS on plasma sodium levels should be explored further to determine whether there is an optimum administration method to achieve a therapeutic hyperosmolar state without resulting in adverse hypernatremia.
Implications for Practice
Despite a lack of clarity regarding the benefits of HTS in the management of acute TBI, a recent practice survey reported that most UK centers are moving to the use of HTS as first-line hyperosmolar therapy over mannitol [25, 26]. Use of near-patient sodium monitoring (e.g., blood gas analysis) may, in part, make it easier for clinicians to use and titrate HTS. This review shows that there is currently insufficient evidence to make a recommendation for HTS over other ICP-lowering agents in patients with acute TBI. However, it should be noted that this evidence is of low or very low certainty, and any beneficial effect of HTS would need to be balanced against the potential risk of hypernatremia.
Implications for Research
Currently, there is a paucity of large-scale RCT data comparing ICP-lowering agents in the context of TBI. This is partly explained by the relatively rare prevalence of severe TBI necessitating the use of ICP-lowering agents in critical care settings, which imposes limits on trial recruitment. Similarly, there is a lack of available RCT data from lower middle-income countries and pediatric populations. For instance, this review includes only one pediatric study reporting a GOS score [21]. This limits the generalizability of the findings in this review, which includes trials enrolling predominantly adult patients from higher-income countries. There is a need for larger international and multicenter trials in a variety of settings to address the current lack of high-quality evidence and to determine whether there are preferred ICP-lowering therapies in specific patient populations. The ongoing Sugar or Salt phase III trial (including 25–28 ICUs across the United Kingdom) may provide further clarity on benefits or risks associated with the use of HTS in patients with acute TBI (ISRCTN16075091) [26].
Finally, heterogeneous reporting of outcomes after TBI (including long-term functional outcome scores such as the GOS score) across clinical trials compromises the validity of comparison between studies and hinders progress in this field. This review highlights the inconsistency in TBI outcome reporting. For instance, three trials included in narrative syntheses for this review reported GOS scores in forms that were not amenable to inclusion in a pooled analysis. Vialet et al. [23] only reported the number of patients with severe disability or who were deceased at 90 days. Jagannatha et al. [20] defined “favorable” outcome as “good recovery,” “moderate disability,” or “severe disability,” which is likely to be at odds with what most patients would consider to be favorable. Furthermore, Kumar et al. [21] reported the number of patients surviving with or without disability and the number of patients in a vegetative state or deceased by 6 months [21]. These methods of GOS reporting are unlikely to be helpful to clinicians or patients and emphasize the need for a standardized core outcome set for TBI. The core outcome set for trials in significant traumatic brain injury (COSTS-TBI) project aimed to develop a core outcome set to set a standard for future trials including patients with moderate to severe TBI but has since been withdrawn in 2021 [27]. Working toward an international consensus on TBI outcome reporting standards will enable meaningful comparison of trial data worldwide and will allow for better assessment of ICP-lowering therapies in different critical care settings. Further consensus on thresholds for adverse hypernatremia and optimum monitoring of plasma sodium concentration and clinical features in patients receiving HTS will be helpful in the assessment of this outcome.
Strengths and limitations
This review followed a strict methodological process, adhering to Cochrane, PRISMA, and GRADE recommendations. We have also included recently published data from the COBI trial [11], which is the largest RCT investigating the use of HTS for acute TBI to date and has been excluded from previous reviews on this subject [10, 28]. Limitations of this review can be attributed to the clinical and methodological differences between trials, which also included generally small sample sizes. Moreover, differences in outcome reporting methods limited the data suitable for inclusion in meta-analyses and precluded sensitivity and subgroup analyses based on age group, TBI severity, and administration methods. As a result, it is still unclear whether there is an optimum hyperosmolar therapy depending on patient age group or severity of TBI. Additionally, dichotomization of the primary outcome into “favorable” versus “unfavorable” outcomes required us to make judgments about what most patients and clinicians would consider to be a reasonable dichotomy. This was considered necessary to enable meta-analysis because of the variation in GOS reporting across the included trials, and some provided data for pooled GOS scores rather than for each individual GOS score. Thus, it is possible that important information about long-term neurological outcome that could influence or guide patient and clinician decisions is not represented in these findings.
Conclusions
Despite increased popularity in its use, we have shown that there is no evidence of an effect of intravenous HTS compared with other ICP-lowering hyperosmolar agents (mannitol) on important outcomes of interest, including long-term neurological function (measured by GOS score), all-cause mortality, uncontrolled ICP, and length of hospital or ICU stay. HTS may be associated with higher risk of hypernatremia. However, this conclusion is based on very low to low certainty evidence, and clinicians must balance any benefits of HTS with the risk of hypernatremia. In the future, larger well-designed trials investigating the use of hyperosmolar agents in patients with TBI with a comprehensive core outcome set are required to provide further clarity and to guide clinical practice. Overall, these results do not support a recommendation for use of HTS over mannitol in treatment of patients with raised ICP secondary to acute TBI.
References
Corrigan JD, Selassie AW, Orman JA. The epidemiology of traumatic brain injury. J Head Trauma Rehabil. 2010;25:72–80.
National Clinical Guideline C. National Institute for Health and Clinical Excellence: Guidance. Head Injury: Triage, Assessment, Investigation and Early Management of Head Injury in Children, Young People and Adults. London: National Institute for Health and Care Excellence (UK). Copyright © National Clinical Guideline Centre, 2014; 2014.
Sheth KN, Stein DM, Aarabi B, et al. Intracranial pressure dose and outcome in traumatic brain injury. Neurocrit Care. 2013;18:26–32.
Rossi S, Picetti E, Zoerle T, et al. Fluid management in acute brain injury. Curr Neurol Neurosci Rep. 2018;18:74.
Strandvik GF. Hypertonic saline in critical care: a review of the literature and guidelines for use in hypotensive states and raised intracranial pressure. Anaesthesia. 2009;64:990–1003.
Mangat HS, Chiu YL, Gerber LM, Alimi M, Ghajar J, Härtl R. Hypertonic saline reduces cumulative and daily intracranial pressure burdens after severe traumatic brain injury. J Neurosurg. 2015;122:202–10.
Mangat HS, Wu X, Gerber LM, et al. Hypertonic saline is superior to mannitol for the combined effect on intracranial pressure and cerebral perfusion pressure burdens in patients with severe traumatic brain injury. Neurosurgery. 2023;86:221–30.
Carney N, Totten AM, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury. Neurosurgery. 2017;80:6–15.
Froelich M, Ni Q, Wess C, Ougorets I, Härtl R. Continuous hypertonic saline therapy and the occurrence of complications in neurocritically ill patients. Crit Care Med. 2009;37:1433–41.
Chen H, Song Z, Dennis JA. Hypertonic saline versus other intracranial pressure–lowering agents for people with acute traumatic brain injury. Cochrane Database Syst Rev. 2019;12:CD010904.
Roquilly A, Moyer JD, Huet O, et al. Effect of continuous infusion of hypertonic saline vs standard care on 6-month neurological outcomes in patients with traumatic brain injury: the COBI randomized clinical trial. JAMA. 2021;325:2056–66.
Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–4.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Schünemann H BJ, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. 2013.
Cottenceau V, Masson F, Mahamid E, et al. Comparison of effects of equiosmolar doses of mannitol and hypertonic saline on cerebral blood flow and metabolism in traumatic brain injury. J Neurotrauma. 2011;28(10):2003–12.
Francony G, Fauvage B, Falcon D, et al. Equimolar doses of mannitol and hypertonic saline in the treatment of increased intracranial pressure. Crit Care Med. 2008;36(3):795–800.
Harutjunyan L, Holz C, Rieger A, Menzel M, Grond S, Soukup J. Efficiency of 7.2% hypertonic saline hydroxyethyl starch 200/0.5 versus mannitol 15% in the treatment of increased intracranial pressure in neurosurgical patients: a randomized clinical trial [ISRCTN62699180]. Crit Care. 2005;9:R530–40.
Hendoui N, Beigmohammadi MT, Mahmoodpoor A, et al. Reliability of calcium-binding protein S100B measurement toward optimization of hyperosmolal therapy in traumatic brain injury. Eur Rev Med Pharmacol Sci. 2013;17:477–85.
Jagannatha AT, Sriganesh K, Devi BI, Rao GS. An equiosmolar study on early intracranial physiology and long term outcome in severe traumatic brain injury comparing mannitol and hypertonic saline. J Clin Neurosci. 2016;27:68–73.
Kumar SA, Devi BI, Reddy M, Shukla D. Comparison of equiosmolar dose of hyperosmolar agents in reducing intracranial pressure-a randomized control study in pediatric traumatic brain injury. Childs Nerv Syst. 2019;35:999–1005.
Patil H, Gupta R. A comparative study of bolus dose of hypertonic saline, mannitol, and mannitol plus glycerol combination in patients with severe traumatic brain injury. World Neurosurg. 2019;125:e221–8.
Vialet R, Albanèse J, Thomachot L, et al. Isovolume hypertonic solutes (sodium chloride or mannitol) in the treatment of refractory posttraumatic intracranial hypertension: 2 mL/kg 7.5% saline is more effective than 2 mL/kg 20% mannitol. Crit Care Med. 2003;31:1683–7.
Wahdan AS, Al-Madawi AA, El-Shafey KA, Othman SH. Comparison of intermittent versus continuous infusion of 3% hypertonic saline on intracranial pressure in traumatic brain injury using ultrasound assessment of optic nerve sheath. Egypt J Anaesth. 2022;38:291–9.
Wenham TN, Hormis AP, Andrzejowski JC. Hypertonic saline after traumatic brain injury in UK neuro-critical care practice. Anaesthesia. 2008;63:558–9.
Rowland MJ, Veenith T, Scomparin C, et al. Sugar or salt (“SOS”): a protocol for a UK multicentre randomised trial of mannitol and hypertonic saline in severe traumatic brain injury and intracranial hypertension. J Intensive Care Soc. 2022;23:222–32.
A core outcome set for trials in significant traumatic brain injury: COSTS-TBI; 2021.
Mekonnen M, Ong V, Florence TJ, et al. Hypertonic saline treatment in traumatic brain injury: a systematic review. World Neurosurg. 2022;162:98–110.
Acknowledgements
We thank Carolyn Smith (Bodleian Libraries, Oxford, UK) for assisting with development of the search strategy.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
KB, WM, and AS contributed substantially to study design and data analysis and interpretation. MJR provided content expertise. KB conducted the searches. KB and WM performed the data extraction and data analysis. KB drafted the manuscript, and WM, MJR, and AS revised it critically. AS supervised KB and WM for this work. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
MJR is a coinvestigator on the UK Sugar or Salt trial (ISRCTN 16075091), which is comparing hypertonic saline vs. mannitol in patients with traumatic brain injury. The other authors declare no conflicts of interest.
Ethical approval/informed consent
No ethical approval was required for this study as the source data have all been published.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bernhardt, K., McClune, W., Rowland, M.J. et al. Hypertonic Saline Versus Other Intracranial-Pressure-Lowering Agents for Patients with Acute Traumatic Brain Injury: A Systematic Review and Meta-analysis. Neurocrit Care 40, 769–784 (2024). https://doi.org/10.1007/s12028-023-01771-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-023-01771-9