Abstract
Tissue fibrosis is a key factor leading to disability and death worldwide; however, thus far, there are no approved treatments for fibrosis. Transforming growth factor (TGF)-β is a major pro-fibrotic cytokine, which is expected to become a target in the treatment of fibrosis; however, since TGF-β has a wide range of biological functions involving a variety of biological processes in the body, a slight change in TGF-β may have a systematic effect. Indiscriminate inhibition of TGF-β can lead to adverse reactions, which can affect the efficacy of treatment. Therefore, it has become very important to explore how both the TGF-β signaling pathway is inhibited and the safe and efficient TGF-β small molecule inhibitors or neutralizing antibodies are designed in the treatment of fibrotic diseases. In this review, we mainly discuss the key role of the TGF-β signaling pathway in fibrotic diseases, as well as the development of fibrotic drugs in recent years, and explore potential targets in the treatment of fibrotic diseases in order to guide subsequent drug development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to relevant statistics, in the USA, nearly 45% of deaths for patients who suffered from various diseases can be attributed to tissue fibroproliferative diseases [1]. Thus far, there is no approved treatment effective against fibrosis. Although the average life expectancy has been greatly improved as medical technology continues to develop, there is an increasing incidence of organ fibrotic diseases among younger patients and more generalized disease patterns [2,3,4,5,6]. Therefore, there is an urgent need to develop effective drugs for the treatment of fibrosis.
There are many factors leading to fibrosis, including occupation, hereditary disease, lifestyle, aging [7, 8], as well as physical, chemical, and biological factors, etc. [6, 9, 10]. For example, silicosis is a serious occupational disease in China. Due to the long-term exposure to the environment containing silica dust, these particles will accumulate in the lungs and will not be metabolized by the body. Workers who work for a long time will form nodules in the lungs, forming inflammatory lesions and inducing pulmonary fibrosis, and eventually causing irreversible damage to the body [11, 12]. In addition to the above reasons, studies have also reported that the lack of neuraminidase 1(NEU1) is closely related to the formation of fibrosis in muscles, kidneys, liver, heart, and lungs[13]. There is also a genetic link between rheumatoid arthritis-related interstitial lung disease and idiopathic pulmonary fibrosis [14, 15]. Finally, a poor lifestyle is also the main cause of chronic inflammation and even organ fibrosis.
In the process of fibrosis formation, a variety of molecular mechanisms and changes in the microenvironment are involved. For example, TGF-β, platelet-derived growth factor (PDGF), connective tissue growth factor (CTGF), interleukin-4 (IL-4), interleukin-13 (IL-13), and other cytokineshave chemotactic effects on fibroblasts and regulate the synthesis and degradation of collagen, which in turn is closely related to the occurrence and development of fibrosis [16,17,18,19]. In addition, oxidative stress and inflammation can promote the formation of fibrosis [18,19,20,21]. Among the above factors that cause fibrosis, TGF-β plays a vital role. As shown in Fig. 1, TGF-β will be overactivated in persistent inflammation such as tissue damage. The activated TGF-β not only mediates SMAD signaling pathway but also activates the PI3K-AKT-mTOR signaling pathway for transcriptional regulation, which in turn promotes epithelial-mesenchymal transition (EMT) and triggers the accumulation of extracellular matrix [22, 23]. On the other hand, TGF-β can also regulate the activity of RhoA, promoting the activation of Rho-related kinase (ROCK) and inhibiting cofilin (CFL) [24]. Rac and Cdc42 are also involved in the non-Smad signaling pathway mediated by TGF-β signaling [25,26,27,28,29]. Most notably, TGF-β1 is the strongest profibrotic cytokine discovered to date [30,31,32,33,34]; many studies have shown that blocking the TGF-β1 pathway can improve organ fibrosis [32, 35]. However, TGF-β participates in the regulation of a variety of other signal pathways in the body and has an important role in maintaining physiological homeostasis (including immune regulation and tumor suppression) [32]. Therefore, in the drug design process, it is necessary to consider minimizing the potential adverse effects of systemic blocking of TGF-β.
Schematic diagram of TGF-β signaling pathway. A Synthesis of TGF-β precursor and activation of mature TGF β. In the cytoplasm, SLC and LTBP combine to form a large latent complex (LLC) that is secreted into the peripheral circulation of the cells. LTBP can mediate the non-covalent binding of LLC to fibrillin and promote the release of TGF-β by different means such as proteases, integrins, pH, and reactive oxygen species-mediated ways as mentioned above. B Nonclassical Smad signaling pathway mediated by TGF-β. TGF-β phosphorylates downstream adaptor molecules such as RhoA, Ras, TAK1, and P13K and activates the downstream signal amplification cascade including MKKs and MEKs, JNK/SPAK, p38, and other pathways. C Classical Smad signaling pathway mediated by TGF-β. TβRIII presents TGF-β to TβRII, and TβRII combined with TGF-β recruits and phosphorylates TβRI. Finally, the dimerized TβRI and the dimerized TβRII are cross-linked and then trigger the intracellular TGF-β signaling pathway
Judging from the current clinical incidence rate of fibrotic diseases, pulmonary fibrosis, renal fibrosis, and liver fibrosis are the most common fibrotic diseases, and there is an enormous need for the treatment of these diseases clinically which is not being met [36,37,38]. An understanding of fibrotic disease mechanisms has accelerated the research and development of its clinical treatment. Currently, dozens of anti-fibrosis drugs with different targets are under development. The main treatments are listed below. (1) Chemically synthesized oligonucleotides as miRNA inhibitors or analogs. Since studies have shown that some miRNAs could upregulate or downregulate the transcription of specific genes related to fibrosis [39,40,41,42,43]. For example, it was found that the injection of miR-326 into mice with bleomycin-induced pulmonary fibrosis caused significant downregulation of TGF-β1, Smad3, matrix metalloproteinase-9 (MMP-9), and upregulation of Smad7, which in turn have good anti-fibrosis effects [39]. (2) Recombinant serum amyloid P (Pentraxin-2), which has the function of regulating the natural immune response, has the function of inhibiting the differentiation of monocytes into fibroblasts or fibrosis-promoting phenotypes and activating macrophage subpopulations. It is located at the injury site and has a dual effect by inhibiting fibrosis and promoting repair. PRM-151 is a recombinant protein of Pentraxin-2, which has shown good efficacy in the treatment of pulmonary fibrosis and is currently undergoing phase III clinical trials in patients with idiopathic pulmonary fibrosis (IPF) [22, 44]. (3) Another potential therapeutic target is LoxL2 (a lysyl oxidase family member), which plays a key role in ECM cross-linking, affecting the expression of certain specific genes, which are related to fibrosis and carcinogenesis [45,46,47,48,49,50]. GS-6624 is a humanized IgG4 monoclonal antibody that targets LoxL2, blocks the activity of LoxL2, reduces the production of ECM by myofibroblasts, and has shown good efficacy in treating fibrosis [51]. (4) Drugs that target IL-4 and IL-13; IL-4 and IL-13 are also potential targets in the treatment of fibrosis, which are important for mediating innate immune activation and helper T-cell 2 (Th-2) cell response[16]. Studies have shown that IL-13 participates in the generation of TGF-β1 by regulating IL-13 Ralpha2 receptors and promotes the process of fibrosis [52,53,54]. (5) Drugs that target TGF-β [33, 55, 56]. For example, it has been found that the small molecular inhibitor LY364947 selectively inhibits TGF-β1 could effectively block the activation and proliferation of mouse cardiac fibroblasts with fewer side effects and is safer than pan-TGF-β blocking in vivo [33]. In order to avoid harmful effects, choosing appropriate inhibitory strength, drug duration, combination with other drugs that can reduce side effects [57], and local inhibition of TGF-β1 signaling may be good drug development strategies. For example, designing bifunctional antibodies to make the drug more enriched at the lesion site may be a good choice. Since TGF-β has a high degree of pleiotropy, there are still many problems to be resolved in fibrosis treatment by targeting TGF-β. However, it is clear that this cytokine has great therapeutic value. In this paper, we will outline the application and potential of targeting TGF-β in the treatment of tissue fibrosis by introducing advances made in scientific research involving mechanisms that TGF-β may be involved in.
TGF-β superfamily signaling pathway
TGF-β superfamily members
TGF-β superfamily members can regulate the proliferation, differentiation, apoptosis, adhesion, and migration of a variety of cells, such as macrophages, T cells, B cells, immature hematopoietic cells, neutrophils, and dendritic cells, etc. [58,59,60,61]. The TGF-β family consists of 33 members, including TGF-β, growth differentiation factors (GDFs), bone morphogenetic protein (BMP), activin, NODAL, and anti-Mullerian hormone (AMH) [53, 62].
TGF-β has three subtypes in mammals, namely TGF-β1, TGF-β2, and TGF-β3. Although their structures are highly similar, they perform different functions. TGF-β1 plays an important role in maintaining the stability of the body. It can activate fibroblasts and promote the synthesis of the extracellular matrix [63]. Studies have also shown that loss of TGF-β1 may cause defects in hematopoietic and endothelial cell differentiation or autoimmune diseases [64], and lack of TGF-β2 can affect epithelial-mesenchymal interactions, cell growth, extracellular matrix production, and result in tissue remodeling disorders, causing defects in the heart, lungs, cranium and face, limbs, spine, eyes, inner ears, and urogenital system [65]. TGF-β3 plays an important role in the normal morphogenesis of the palate and lungs and participates in epithelial-mesenchymal interaction [66], in which it can reduce scar formation during wound healing [67]. Among the three TGF-β subtypes, TGF-β1 is the most fully studied member of the transforming growth factor family, which plays a major role in tumor development and tissue fibrosis.
TGF-β receptors
TGF-β receptors are divided into three categories: transforming growth factor-β type I receptor (TβRI), transforming growth factor-β type II receptor (TβRII), and transforming growth factor-β type III receptor (TβRIII)[68, 69]. TβRII contains a serine/threonine-rich sequence, which can undergo autophosphorylation. TβRI contains a conservative serine/glycine-rich sequence (TTSGSGSGLP, also known as GS region), which plays a key part in TβRI activation. Both TβRI and TβRII can directly participate in the signal transmission process [69]. TβRIII is a co-receptor of the TGF-β superfamily. The extracellular domain of TβRIII contains an independent amino terminal domain, a zona pelucida domain (ZPD) potentially involved in the oligomerization reaction of the receptor, and two independent TGF-β ligand-binding domains [70]. TβRIII can mediate both the classic Smad signaling pathway and the non-classical Smad signaling pathway to regulate the downstream signaling of TGF-β [71,72,73] and plays a role in ligand presentation in the TGF-β classic signaling pathway. However, in many diseases, TβRIII can block the TGF-β signaling pathway by forming a complex with TβRI and TβRII [74]. In the classical TGF-β pathway, active TGF-β can first bind to TβRIII, then be presented to the receptor complex composed of TβRI and TβRII, and initiate downstream signaling pathways (Fig. 1) [75, 76].
TGF-β secretion and activation
The TGF-β family members are composed of latency-related peptide (LAP), precursor domain, and C-terminal TGF-β fragment, where the precursor domain and growth factor are connected by a cleavage site of proprotein convertase (PC) [77, 78]. The TGF-β precursor is first synthesized on the rough endoplasmic reticulum [79]. After being transferred into the Golgi complex and cleaved by the invertase furin, it is bound with LAP homodimer in a non-covalent form and forms a symmetrical heterotetrameric structure, which is called small latent complex (SLC) [79]. This SLC functions as a cover to prevent mature TGF-β from binding to cell surface receptors. SLC can be hydrolyzed by protease or non-protease to form mature TGF-β with biological activity [80, 81]. During the process, the homodimer part of LAP is connected by two disulfide bonds, and the interaction of the mature TGF-β part is also stabilized by disulfide bonds [82].
The activation of latent TGF-β complex involves several important molecular mechanisms as shown in Fig. 1. (1) The arginine-glycine-aspartate (RGD) motif in TGF-β precursor fragment bound with integrin β chain via non-covalent bonds. When integrin αvβ6/αvβ8 on stromal cells binds to RGD sequence on the TGF-β precursor domain, under the combined action of mechanical force of cytoskeleton and the reaction force of the extracellular matrix or cells presenting TGF-β, the closed loop of precursor domain opens and mature TGF-β with biological function is released [79, 81, 83]. (2) The TGF-β precursor fragment is cleaved by proteases such as metalloprotease MMP-2, MMP-3, MMP-9, and plasmin to release mature TGF-β [79, 84]. (3) Reactive oxygen species (ROS); in the process of liver fibrosis, activated hepatic stellate cells release a large amount of ROS, and a high level of ROS can promote the release of transforming growth factor-β [85]. (4) Changes in pH; in a tissue microenvironment with a low pH, mature TGF-β is released more easily [54, 86, 87]. (5) Studies have shown that the small latent complex can also bind to glycoprotein-A repetitions predominant (GARP, usually overexpressed on the membrane of the Treg cells) on cell surfaces, leading to the release of active TGF-β and the regulation of the proliferation and differentiation of Treg cells [80, 83] (Fig. 1).
TGF-β signaling pathway
Once activated, mature TGF-β initiates transmembrane signaling by binding itself to two distinct transmembranes Ser/Thr protein kinases, termed as TβRI and TβRII receptors. Among them, transforming growth factor-β can also bind to the helper receptor TβRIII, and then the signal molecules are transmitted to TβRI and TβRII by T-β RIII. TβRII can phosphorylate specific cytoplasmic GS domains of TβRI, leading to conformational regulation of TβRI so as to phosphorylate Smad2 and Smad3 proteins. Subsequently, phosphorylated Smad2 and Smad3 proteins bind to Smad4 protein to form heterologous complexes, with the formed heterotrimers translocating into the nucleus and bound to DNA to regulate the transcription of multiple target genes [62, 88,89,90]. In the nucleus, with the participation of certain DNA-binding proteins, Smad molecules act on specific target genes to regulate their expression. For example, phosphorylated Smad2/3/4 heterocomplexes can form complexes with p300 and CREB-binding proteins (CBP) to promote the transcription of target genes such as plasminogen activator inhibitor-1 (PAI-1), collagen type I alpha 1 (COL1A1), and connective tissue growth factor (CTGF) [91] to regulate the production of the extracellular matrix. In addition to interacting with transcription factors, Smad complexes have been shown to regulate epigenetic modifications by transferring histone acetyl and methyltransferases to specific gene sites. Phosphorylated Smad2/3 protein can bind to M6A methyltransferase complex to regulate m6A RNA methylation modification of target genes, thus exerting the biological function of DNA repair and posttranscriptional regulation [62, 92].
More and more pieces of evidence clearly demonstrate that in addition to activating classic Smad-dependent signaling, TGF-β also participates in nonclassical signaling pathways, which mainly include MAP kinases, PI-3 kinase-Akt, and Rho-like GTPase [93,94,95,96]. Figure 1 depicts the classic TGF-β signaling pathway and nonclassical TGF-β signaling pathway.
The role of TGF-β in the progression of tissue fibrosis
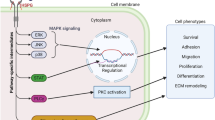
It has been well known that fibrosis is not a disease but a result of tissue damage repair dysfunction [54]. TGF-β plays an important role in this process. During tissue damage repair, mesenchymal cells undergo significant metabolic changes to promote energy-consuming cell functions including cell proliferation and protein synthesis [97,98,99]. The increased glycolysis activity of fibroblasts results in the synthesis of pyruvic acid and lactic acid. The generated lactic acid reduces extracellular pH, which induces the activation of potential TGF-β1 [54, 100]. Meanwhile, integrin-mediated TGF-β activation promotes the expression of IL-17A, which increases the expression of TGF-β receptors in fibroblasts, thereby promoting the response of fibroblasts to TGF-β signals [54, 101,102,103]. In addition, in fibroblasts stimulated by TGF-β1, the increase in the level of glutaminase promotes the decomposition of glutamine, endows the cells with antiapoptotic properties [104], finally promotes the production and stability of collagen through mTOR signal transduction [105]. Studies have shown that inhibiting glutaminase I can improve the symptoms of pulmonary fibrosis induced by bleomycin and TGF-β1 in vivo [106]. And TGF-β is one of the main regulators of cell differentiation, migration, proliferation, and gene expression [107]. In injured or diseased tissues, sustained, dysregulated, or hyperactive TGF-β transcriptional activation leads to enhanced fibrogenesis, which impairs normal tissue regeneration and may cause dysfunction by interfering with the structure of organ structural units [108]. The specific cell source of TGF-β in the body is unclear. Epithelial cells, platelets, T cells, fibroblasts, and mast cells can all produce TGF-β [109,110,111,112]. In the study of acute and chronic fibrotic injury models, it was found that disrupting the pathways involved in the recruitment of macrophages can reduce the synthesis of TGF-β and relieve fibrosis, which indicates that macrophages are also an important source of TGF-β [113, 114]. The continuous increase in TGF-β will further deepen the degree of fibrosis and form a serious vicious circle (Fig. 2).
Anti-fibrosis drug research
Fibrosis is a common manifestation of chronic tissue damage. A further understanding of the mechanisms of fibrotic diseases will help accelerate the development of its clinical treatment. At present, the main mechanism of action used by drugs under development against fibrosis is related to the inhibition of various factors in the formation of fibrosis, including inhibition of the signaling of cytokines such as TGF-β, PDGF, and CTGF, inhibition of fibroblast division and proliferation, regulation of collagen synthesis and degradation, and regulation of oxidative stress, inflammation, and other reaction processes that contribute to the formation of fibrosis [17, 38, 54, 115]. For example, HSC activation is a key step in the formation of liver fibrosis, so HSC is regarded as an important target in the development of drugs used against liver fibrosis [44]. Since the discovery of the importance of TGF-β in fibrotic diseases, drug research targeting the TGF-β signaling pathway has increased significantly [58, 67, 90]. Related drug types include antisense oligonucleotides (AON), neutralizing antibodies, cyclic RGD pentapeptides, TGF-β ligand traps, and small molecule kinase inhibitors (SKIs), etc.[80, 116,117,118]. In early anti-fibrosis drug research, researchers found that pan-TGF-β antibody drugs might cause cardiotoxicity [54, 119]. Through the mouse model experiments, it was found that the cardiotoxicity caused by pan-TGF-β antibody drugs may be related to the indiscriminate blocking of TGF-β2 and TGF-β3 signaling pathways [119], the results also provide guidance for the design of selective targeting of TGF-β drugs. There are already a variety of drugs targeting TGF-β superfamily members or their receptors under development, such as the highly selective antibody SRK-181 developed by Scholar Rock, which targets the TGF-β1 precursor. The main mechanism of SRK-181 is to prevent the cleavage of TGF-β1 precursor and release mature TGF-β by binding to TGF-β1 precursor [120]. In the 4-week repeated-dose rat toxicity study of SRK-181 (the highest dose was 100 mg/kg, which was much higher than the dose required to induce a strong antitumor response in combination with PD-1 antibody), the researchers did not observe other histological features of cardiac valvulopathy or cardiotoxicity. This indicates that selective blocking of TGF-β1 activation may avoid the dose-limiting toxicity caused by the indiscriminate TGF-β inhibitor drugs to a certain extent [119, 121]. The AVID200 developed by Forbius has also attracted widespread attention [122, 123]. AVID200 is a highly effective and selective inhibitor specifically targeting TGFβRII mutant that enhances the binding activity of TGFβRII to TGF-β1 and TGF-β3 and thus greatly reduces the binding activity to TGF-β2 [123]. In clinical phase I trials, researchers found that AVID200 has a good anti-fibrosis effect, and preclinical models showed that blocking TGF-β signal transduction can reverse myelofibrosis and restore hematopoietic function without safety risks [122,123,124]. Other than that, a polypeptide drug HTPEP-001 targeting TGF-β1 for the treatment of IPF, which was developed by Chengdu Huitai Biotechnology has also shown good results in preclinical experiments [125]. The study found that by inhibiting the production of active TGF-β1 and Smad signaling, aerosol inhalation of HTPEP-001 effectively blocked the fibrosis process in the rat model of bleomycin-induced pulmonary fibrosis, and there were no obvious adverse events related to immunological or histological changes [125]. The above studies all support selective targeting TGF-β as a promising direction for the treatment of fibrotic diseases. The table below summarizes some of the drugs under development for the treatment of fibrotic diseases (Table 1).
It is well known that the tissues surrounding the tumor microenvironment are rich in dense fibrotic cells, which are usually referred to as cancer-associated fibroblasts (CAF) [126]. Therefore, the occurrence and development of tumors are closely related to fibrosis. The progress of drugs targeting TGF-β for tumor therapy under development is also summarized in Table 1. More and more drugs targeting TGF-β are being developed to treat diseases such as fibrosis and tumors, which expand our understanding of the TGF-β signaling pathway and its mechanism of action and further promote the development of new anti-fibrotic drugs targeting TGF-β with few or no side effects.
Conclusions
With increasing in-depth research on the pathogenesis of fibrotic diseases, it has been shown that the TGF-β signaling pathway is closely related to organ fibrosis [54, 57, 67, 83, 91]. Since TGF-β participates in the regulation of multiple signaling pathways in the body, and TGF-β is closely related to the body’s metabolism, aging, circadian rhythm, epigenetics, EMT, and other cellular processes, learning how to regulate the TGF-β signaling pathway for the treatment of fibrosis while avoiding toxic side effects has become key to drug development [68, 127,128,129]. At present, by targeting different action sites of TGF-β and receptors, the search, design, and screening of various efficient and low-toxic novel small molecule inhibitors have become a research hotspot. Although some preclinical and clinical drug candidates for blocking TGF-β signaling pathway exhibit some side effects, such as pirfenidone (drug details are shown in Table 1), its anti-fibrosis effect is still quite encouraging [130], which provides some enlightenment for the development of new inhibitors of the TGF-β signaling pathway. However, a single drug often leads to obvious adverse reactions due to factors such as a single target and a large dose; hence, combination therapy has become the treatment trend of fibrosis disease. In addition, the use of the abovementioned inhibitors targeting the TGF-β signaling pathway reasonably combined with other drugs of different action mechanisms to treat a variety of fibrosis-related diseases has also attracted the attention of many researchers. In short, with the continuous improvement of drug development strategies and the increasing number of safe and effective small molecule inhibitors, it is believed that more effective drugs targeting TGF-β signaling for the treatment of fibrosis will enter the clinical practice.
Since a group of abnormal proliferation cells with high fibrotic characteristics also exists in most tumor microenvironments in addition to being a popular target for fibrotic diseases, TGF-β signaling is also one of the most popular targets in the field of tumor immunotherapy in recent years [97]. The bifunctional antibody drug M7824, which is being developed by Merck, has attracted widespread attention in the pharmaceutical industry due to its good synergistic therapeutic mechanism for tumors [131, 132]. The drug can effectively reduce the formation of cell matrix around the tumor tissue by targeting TGF-β and anti-PD-L1 to further promote the penetration of T cells into the center of the tumor and trigger a more effective anti-tumor immune effect [131,132,133]. It also has fewer adverse effects on the tumor microenvironment and is safer compared with pan-TGF-β blocking. In this review, we illuminate that the specific blocking of TGF-β1 is a good way to avoid the uncertainty caused by the indiscriminate suppression of TGF-β signal pathways. However, we still face great challenges in the future. For example, how to design and screen drugs targeting TGF-β1 and how to further reduce or eliminate the side effects of targeted drugs. All of these problems require more in-depth thinking and more effective solution strategies.
References
Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4(8):583–94. https://doi.org/10.1038/nri1412.
Barber CM, Fishwick D. Importance of past occupational exposures in the rising incidence of idiopathic pulmonary fibrosis in the U.K. Thorax. 2012;67(3):264. https://doi.org/10.1136/thoraxjnl-2011-200836.
Navaratnam V, Fleming KM, West J, et al. The rising incidence of idiopathic pulmonary fibrosis in the U.K. Thorax. 2011;66(6):462–7. https://doi.org/10.1136/thx.2010.148031.
Lai CC, Wang CY, Lu HM, et al. Idiopathic pulmonary fibrosis in Taiwan - a population-based study. Respir Med. 2012;106(11):1566–74. https://doi.org/10.1016/j.rmed.2012.07.012.
Mocumbi AO, Ferreira MB, Sidi D, et al. A population study of endomyocardial fibrosis in a rural area of Mozambique. N Engl J Med. 2008;359(1):43–9. https://doi.org/10.1056/NEJMoa0708629.
Agrawal A, Agarwal A, Mehta D, et al. Nationwide trends of hospitalizations for cystic fibrosis in the United States from 2003 to 2013. Intractable & rare diseases research. 2017;6(3):191–8. https://doi.org/10.5582/irdr.2017.01043.
Krizhanovsky V, Yon M, Dickins RA, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134(4):657–67. https://doi.org/10.1016/j.cell.2008.06.049.
Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nature Reviews Molecular Cell Biology. 2007;8(9):729–40. https://doi.org/10.1038/nrm2233.
Ray JG, Chatterjee R, Chaudhuri K. Oral submucous fibrosis: a global challenge. Rising incidence, risk factors, management, and research priorities. Periodontology 2000. 2019;80(1):200–12. https://doi.org/10.1111/prd.12277.
Schneider KM, Mohs A, Kilic K, et al. Intestinal microbiota protects against MCD diet-induced steatohepatitis. International Journal of Molecular Sciences. 2019;20(2). https://doi.org/10.3390/ijms20020308
Krefft S, Wolff J, Rose C. Silicosis: an update and guide for clinicians. Clin Chest Med. 2020;41(4):709–22. https://doi.org/10.1016/j.ccm.2020.08.012.
Shtraichman O, Kramer MR. Artificial stone silicosis: the Israel epidemic, current view. Harefuah. 2017;156(8):517–21.
van de Vlekkert D, Demmers J, Nguyen X, et al. Excessive exosome release is the pathogenic pathway linking a lysosomal deficiency to generalized fibrosis. Science advances. 2019;5(7):eaav70. https://doi.org/10.1126/sciadv.aav3270.
Seibold MA, Wise AL, Speer MC, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364(16):1503–12. https://doi.org/10.1056/NEJMoa1013660.
Makino S. Progressive fibrosing interstitial lung diseases: a new concept and indication of nintedanib. Mod Rheumatol. 2021;31(1):13–9. https://doi.org/10.1080/14397595.2020.1826665.
Su S, Zhao Q, He C, et al. miR-142-5p and miR-130a-3p are regulated by IL-4 and IL-13 and control profibrogenic macrophage program. Nat Commun. 2015;6:8523. https://doi.org/10.1038/ncomms9523.
Parola M, Pinzani M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37–55. https://doi.org/10.1016/j.mam.2018.09.002.
Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp Biol Med (Maywood). 2008;233(2):109–22. https://doi.org/10.3181/0707-mr-190.
Ma J, Sanchez-Duffhues G, Goumans MJ, et al. TGF-β-Induced endothelial to mesenchymal transition in disease and tissue engineering. Frontiers in cell and developmental biology. 2020;8:260. https://doi.org/10.3389/fcell.2020.00260.
Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al]. 2017;43(Suppl 1):S3-s18. https://doi.org/10.1097/dss.0000000000000819.
Ahmed S, Misra DP, Agarwal V. Interleukin-17 pathways in systemic sclerosis-associated fibrosis. Rheumatol Int. 2019;39(7):1135–43. https://doi.org/10.1007/s00296-019-04317-5.
Raghu G, van den Blink B, Hamblin MJ, et al. Long-term treatment with recombinant human pentraxin 2 protein in patients with idiopathic pulmonary fibrosis: an open-label extension study. Lancet Respir Med. 2019;7(8):657–64. https://doi.org/10.1016/s2213-2600(19)30172-9.
Cho N, Razipour SE, McCain ML. Featured article: TGF-β1 dominates extracellular matrix rigidity for inducing differentiation of human cardiac fibroblasts to myofibroblasts. Exp Biol Med (Maywood). 2018;243(7):601–12. https://doi.org/10.1177/1535370218761628.
Knipe RS, Probst CK, Lagares D, et al. The Rho kinase isoforms ROCK1 and ROCK2 each contribute to the development of experimental pulmonary fibrosis. Am J Respir Cell Mol Biol. 2018;58(4):471–81. https://doi.org/10.1165/rcmb.2017-0075OC.
Xiu A, Ding Q, Li Z, et al. Doxazosin attenuates liver fibrosis by inhibiting autophagy in hepatic stellate cells via activation of the PI3K/Akt/mTOR signaling pathway. Drug Des Dev Ther. 2021;15:3643–59. https://doi.org/10.2147/dddt.S317701.
Xue Y, Zhang M, Liu M, et al. via8-Gingerol ameliorates myocardial fibrosis by attenuating reactive oxygen species, apoptosis, and autophagy the PI3K/Akt/mTOR signaling pathway. Front Pharmacol. 2021;12: 711701. https://doi.org/10.3389/fphar.2021.711701.
Lei H, Wu D, Wang J, et al. C1q/tumor necrosis factor-related protein-6 attenuates post-infarct cardiac fibrosis by targeting RhoA/MRTF-A pathway and inhibiting myofibroblast differentiation. Basic Res Cardiol. 2015;110(4):35. https://doi.org/10.1007/s00395-015-0492-7.
Ge J, Burnier L, Adamopoulou M, et al. RhoA, Rac1, and Cdc42 differentially regulate αSMA and collagen I expression in mesenchymal stem cells. J Biol Chem. 2018;293(24):9358–69. https://doi.org/10.1074/jbc.RA117.001113.
Fritsch R, de Krijger I, Fritsch K, et al. Ras and Rho families of GTPases directly regulate distinct phosphoinositide 3-kinase isoforms. Cell. 2013;153(5):1050–63. https://doi.org/10.1016/j.cell.2013.04.031.
Walton KL, Johnson KE, Harrison CA. Targeting TGF-β mediated SMAD signaling for the prevention of fibrosis. Front Pharmacol. 2017;8:461. https://doi.org/10.3389/fphar.2017.00461.
Yoshida K, Matsuzaki K. Differential regulation of TGF-β/Smad signaling in hepatic stellate cells between acute and chronic liver injuries. Front Physiol. 2012;3:53. https://doi.org/10.3389/fphys.2012.00053.
Kim KK, Sheppard D, Chapman HA. TGF-β1 signaling and tissue fibrosis. Cold Spring Harbor perspectives in biology. 2018;10(4). https://doi.org/10.1101/cshperspect.a022293
Ding J, Tang Q, Luo B, et al. Klotho inhibits angiotensin II-induced cardiac hypertrophy, fibrosis, and dysfunction in mice through suppression of transforming growth factor-β1 signaling pathway. Eur J Pharmacol. 2019;859: 172549. https://doi.org/10.1016/j.ejphar.2019.172549.
Khalil H, Kanisicak O, Prasad V, et al. Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac fibrosis. J Clin Investig. 2017;127(10):3770–83. https://doi.org/10.1172/jci94753.
Klass BR, Grobbelaar AO, Rolfe KJ. Transforming growth factor beta1 signalling, wound healing and repair: a multifunctional cytokine with clinical implications for wound repair, a delicate balance. Postgrad Med J. 2009;85(999):9–14. https://doi.org/10.1136/pgmj.2008.069831.
Glassberg M. Overview of idiopathic pulmonary fibrosis, evidence-based guidelines, and recent developments in the treatment landscape. Am J Manag Care. 2019;25:S95–203.
Wang Y, Xing QQ, Tu JK, et al. Involvement of hydrogen sulfide in the progression of renal fibrosis. Chin Med J. 2019;132(23):2872–80. https://doi.org/10.1097/cm9.0000000000000537.
Roehlen N, Crouchet E, Baumert TF. Liver fibrosis: mechanistic concepts and therapeutic perspectives. Cells. 2020;9(4). https://doi.org/10.3390/cells9040875
Das S, Kumar M, Negi V, et al. MicroRNA-326 regulates profibrotic functions of transforming growth factor-β in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2014;50(5):882–92. https://doi.org/10.1165/rcmb.2013-0195OC.
Liang H, Xu C, Pan Z, et al. The antifibrotic effects and mechanisms of microRNA-26a action in idiopathic pulmonary fibrosis. Molecular therapy : the journal of the American Society of Gene Therapy. 2014;22(6):1122–33. https://doi.org/10.1038/mt.2014.42.
Yang S, Cui H, Xie N, et al. miR-145 regulates myofibroblast differentiation and lung fibrosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27(6):2382–91. https://doi.org/10.1096/fj.12-219493.
Loboda A, Sobczak M, Jozkowicz A, et al. TGF-β1/Smads and miR-21 in Renal fibrosis and inflammation. Mediators Inflamm. 2016;2016:8319283. https://doi.org/10.1155/2016/8319283.
Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11(4):252–63. https://doi.org/10.1038/nrm2868.
Tzouvelekis A, Tzilas V, Antoniou KM, et al. Human pentraxin 2 protein treatment for IPF. Lancet Respir Med. 2019;7(8):640–1. https://doi.org/10.1016/s2213-2600(19)30173-0.
Zhao W, Yang A, Chen W, et al. Inhibition of lysyl oxidase-like 1 (LOXL1) expression arrests liver fibrosis progression in cirrhosis by reducing elastin crosslinking. Biochimica et biophysica acta Molecular basis of disease. 2018;1864(4 Pt A):1129–37. https://doi.org/10.1016/j.bbadis.2018.01.019.
Liu SB, Ikenaga N, Peng ZW, et al. Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016;30(4):1599–609. https://doi.org/10.1096/fj.14-268425.
Aumiller V, Strobel B, Romeike M, et al. Comparative analysis of lysyl oxidase (like) family members in pulmonary fibrosis. Sci Rep. 2017;7(1):149. https://doi.org/10.1038/s41598-017-00270-0.
Dongiovanni P, Meroni M, Baselli GA, et al. Insulin resistance promotes lysyl oxidase like 2 induction and fibrosis accumulation in non-alcoholic fatty liver disease. Clinical science (London, England : 1979). 2017;131(12):1301–15. https://doi.org/10.1042/cs20170175
Harlow CR, Wu X, van Deemter M, et al. Targeting lysyl oxidase reduces peritoneal fibrosis. PLoS ONE. 2017;12(8): e0183013. https://doi.org/10.1371/journal.pone.0183013.
Ikenaga N, Peng ZW, Vaid KA, et al. Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut. 2017;66(9):1697–708. https://doi.org/10.1136/gutjnl-2016-312473.
Chen W, Yang A, Jia J, et al. Lysyl oxidase (LOX) family members: rationale and their potential as therapeutic targets for liver fibrosis. Hepatology (Baltimore, MD). 2020;72(2):729–41. https://doi.org/10.1002/hep.31236.
Fichtner-Feigl S, Strober W, Kawakami K, et al. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12(1):99–106. https://doi.org/10.1038/nm1332.
Diaz A, Tan K, He H, et al. Keloid lesions show increased IL-4/IL-13 signaling and respond to Th2-targeting dupilumab therapy. Journal of the European Academy of Dermatology and Venereology : JEADV. 2020;34(4):e161–4. https://doi.org/10.1111/jdv.16097.
Henderson NC, Rieder F, Wynn TA. Fibrosis: from mechanisms to medicines. Nature. 2020;587(7835):555–66. https://doi.org/10.1038/s41586-020-2938-9.
Giannelli G, Mikulits W, Dooley S, et al. The rationale for targeting TGF-β in chronic liver diseases. Eur J Clin Invest. 2016;46(4):349–61. https://doi.org/10.1111/eci.12596.
Liu L, Shi Q, Liu X, et al. Attenuation of myocardial fibrosis using molecular hydrogen by inhibiting the TGF-β signaling pathway in spontaneous hypertensive rats. Am J Hypertens. 2021. https://doi.org/10.1093/ajh/hpab159.
Rosenbloom J, Ren S, Macarak E. New frontiers in fibrotic disease therapies: the focus of the Joan and Joel Rosenbloom Center for Fibrotic Diseases at Thomas Jefferson University. Matrix biology : journal of the International Society for Matrix Biology. 2016;51:14–25. https://doi.org/10.1016/j.matbio.2016.01.011.
Hu HH, Chen DQ, Wang YN, et al. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem Biol Interact. 2018;292:76–83. https://doi.org/10.1016/j.cbi.2018.07.008.
Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discovery. 2012;11(10):790–811. https://doi.org/10.1038/nrd3810.
Eser P, Jänne PA. TGFβ pathway inhibition in the treatment of non-small cell lung cancer. Pharmacol Ther. 2018;184:112–30. https://doi.org/10.1016/j.pharmthera.2017.11.004.
Nickel J, Ten Dijke P, Mueller TD. TGF-β family co-receptor function and signaling. Acta Biochim Biophys Sin. 2018;50(1):12–36. https://doi.org/10.1093/abbs/gmx126.
Derynck R, Budi EH. Specificity, versatility, and control of TGF-beta family signaling. Sci Signal. 2019;12(570). https://doi.org/10.1126/scisignal.aav5183
Xu F, Liu C, Zhou D, et al. TGF-β/SMAD pathway and its regulation in hepatic fibrosis. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2016;64(3):157–67. https://doi.org/10.1369/0022155415627681.
Dickson MC, Martin JS, Cousins FM, et al. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development (Cambridge, England). 1995;121(6):1845–54.
Sanford LP, Ormsby I, Gittenberger-de Groot AC, et al. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development (Cambridge, England). 1997;124(13):2659–70.
Kaartinen V, Voncken JW, Shuler C, et al. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet. 1995;11(4):415–21. https://doi.org/10.1038/ng1295-415.
Lichtman MK, Otero-Vinas M, Falanga V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair and Regeneration. 2016;24(2):215–22. https://doi.org/10.1111/wrr.12398.
Peng C. The TGF-beta superfamily and its roles in the human ovary and placenta. Journal of Obstetrics and Gynaecology Canada. 2003;25(10):834–44. https://doi.org/10.1016/s1701-2163(16)30674-0.
Chen W, Wahl SM. TGF-beta: receptors, signaling pathways and autoimmunity. Curr Dir Autoimmun. 2002;5:62–91. https://doi.org/10.1159/000060548.
Gatza CE, Oh SY, Blobe GC. Roles for the type III TGF-beta receptor in human cancer. Cell Signal. 2010;22(8):1163–74. https://doi.org/10.1016/j.cellsig.2010.01.016.
Chaikuad A, Bullock AN. Structural basis of intracellular TGF-β signaling: receptors and Smads. Cold Spring Harbor Perspectives in Biology. 2016;8(11). https://doi.org/10.1101/cshperspect.a022111
López-Casillas F, Cheifetz S, Doody J, et al. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell. 1991;67(4):785–95. https://doi.org/10.1016/0092-8674(91)90073-8.
Mendoza V, Vilchis-Landeros MM, Mendoza-Hernández G, et al. Betaglycan has two independent domains required for high affinity TGF-beta binding: proteolytic cleavage separates the domains and inactivates the neutralizing activity of the soluble receptor. Biochemistry. 2009;48(49):11755–65. https://doi.org/10.1021/bi901528w.
Tazat K, Hector-Greene M, Blobe GC, et al. TβRIII independently binds type I and type II TGF-β receptors to inhibit TGF-β signaling. Mol Biol Cell. 2015;26(19):3535–45. https://doi.org/10.1091/mbc.E15-04-0203.
Beck SE, Jung BH, Fiorino A, et al. Bone morphogenetic protein signaling and growth suppression in colon cancer. Am J Physiol Gastrointest Liver Physiol. 2006;291(1):G135–45. https://doi.org/10.1152/ajpgi.00482.2005.
López-Casillas F, Wrana JL, Massagué J. Betaglycan presents ligand to the TGF beta signaling receptor. Cell. 1993;73(7):1435–44. https://doi.org/10.1016/0092-8674(93)90368-z.
Lodyga M, Hinz B. TGF-β1 - a truly transforming growth factor in fibrosis and immunity. Semin Cell Dev Biol. 2020;101:123–39. https://doi.org/10.1016/j.semcdb.2019.12.010.
Gómez-Gil V. Therapeutic implications of TGFβ in cancer treatment: a systematic review. Cancers. 2021;13(3). https://doi.org/10.3390/cancers13030379
Robertson IB, Rifkin DB. Regulation of the bioavailability of TGF-β and TGF-β-related proteins. Cold Spring Harbor perspectives in biology. 2016;8(6). https://doi.org/10.1101/cshperspect.a021907
Neuzillet C, Tijeras-Raballand A, Cohen R, et al. Targeting the TGFβ pathway for cancer therapy. Pharmacol Ther. 2015;147:22–31. https://doi.org/10.1016/j.pharmthera.2014.11.001.
Buscemi L, Ramonet D, Klingberg F, et al. The single-molecule mechanics of the latent TGF-β1 complex. Current biology : CB. 2011;21(24):2046–54. https://doi.org/10.1016/j.cub.2011.11.037.
Gray AM, Mason AJ. Requirement for activin A and transforming growth factor–beta 1 pro-regions in homodimer assembly. Science (New York, NY). 1990;247(4948):1328–30. https://doi.org/10.1126/science.2315700.
Zhang YE. Mechanistic insight into contextual TGF-β signaling. Curr Opin Cell Biol. 2018;51:1–7. https://doi.org/10.1016/j.ceb.2017.10.001.
Maeda S, Dean DD, Gomez R, et al. The first stage of transforming growth factor beta1 activation is release of the large latent complex from the extracellular matrix of growth plate chondrocytes by matrix vesicle stromelysin-1 (MMP-3). Calcif Tissue Int. 2002;70(1):54–65. https://doi.org/10.1007/s002230010032.
Zhang D, Jin W, Wu R, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 2019;51(4):671-81.e5. https://doi.org/10.1016/j.immuni.2019.08.001.
Kottmann RM, Kulkarni AA, Smolnycki KA, et al. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-β. Am J Respir Crit Care Med. 2012;186(8):740–51. https://doi.org/10.1164/rccm.201201-0084OC.
Lin L, Gong H, Li R, et al. Nanodrug with ROS and pH dual-sensitivity ameliorates liver fibrosis via multicellular regulation. Advanced Science (Weinheim, Baden-Wurttemberg, Germany). 2020;7(7):1903138. https://doi.org/10.1002/advs.201903138.
Muraoka RS, Dumont N, Ritter CA, et al. Blockade of TGF-β inhibits mammary tumor cell viability, migration, and metastases. J Clin Investig. 2002;109(12):1551–9. https://doi.org/10.1172/jci200215234.
Carthy JM. TGFβ signaling and the control of myofibroblast differentiation: implications for chronic inflammatory disorders. J Cell Physiol. 2018;233(1):98–106. https://doi.org/10.1002/jcp.25879.
Samarakoon R, Overstreet JM, Higgins PJ. TGF-β signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal. 2013;25(1):264–8. https://doi.org/10.1016/j.cellsig.2012.10.003.
Piersma B, Bank RA, Boersema M. Signaling in fibrosis: TGF-β, WNT, and YAP/TAZ converge. Front Med. 2015;2:59. https://doi.org/10.3389/fmed.2015.00059.
Bertero A, Brown S, Madrigal P, et al. The SMAD2/3 interactome reveals that TGFβ controls m(6)A mRNA methylation in pluripotency. Nature. 2018;555(7695):256–9. https://doi.org/10.1038/nature25784.
Luo K. Signaling cross talk between TGF-β/Smad and other signaling pathways. Cold Spring Harbor perspectives in biology. 2017;9(1). https://doi.org/10.1101/cshperspect.a022137
Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19(1):128–39. https://doi.org/10.1038/cr.2008.328.
Wenner CE, Yan S. Biphasic role of TGF-beta1 in signal transduction and crosstalk. J Cell Physiol. 2003;196(1):42–50. https://doi.org/10.1002/jcp.10243.
Atfi A, Djelloul S, Chastre E, et al. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in transforming growth factor beta-mediated signaling. J Biol Chem. 1997;272(3):1429–32. https://doi.org/10.1074/jbc.272.3.1429.
Batlle E, Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity. 2019;50(4):924–40. https://doi.org/10.1016/j.immuni.2019.03.024.
Hsu HS, Liu CC, Lin JH, et al. Involvement of ER stress, PI3K/AKT activation, and lung fibroblast proliferation in bleomycin-induced pulmonary fibrosis. Sci Rep. 2017;7(1):14272. https://doi.org/10.1038/s41598-017-14612-5.
Shah D, Mital K. The role of trypsin:chymotrypsin in tissue repair. Adv Ther. 2018;35(1):31–42. https://doi.org/10.1007/s12325-017-0648-y.
Xie N, Tan Z, Banerjee S, et al. Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. Am J Respir Crit Care Med. 2015;192(12):1462–74. https://doi.org/10.1164/rccm.201504-0780OC.
Park MJ, Moon SJ, Lee EJ, et al. IL-1-IL-17 signaling axis contributes to fibrosis and inflammation in two different murine models of systemic sclerosis. Front Immunol. 2018;9:1611. https://doi.org/10.3389/fimmu.2018.01611.
Wilson MS, Madala SK, Ramalingam TR, et al. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 2010;207(3):535–52. https://doi.org/10.1084/jem.20092121.
Meng F, Wang K, Aoyama T, et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143(3):765-76.e3. https://doi.org/10.1053/j.gastro.2012.05.049.
Bai L, Bernard K, Tang X, et al. Glutaminolysis epigenetically regulates antiapoptotic gene expression in idiopathic pulmonary fibrosis fibroblasts. Am J Respir Cell Mol Biol. 2019;60(1):49–57. https://doi.org/10.1165/rcmb.2018-0180OC.
Ge J, Cui H, Xie N, et al. Glutaminolysis promotes collagen translation and stability via α-ketoglutarate-mediated mTOR activation and proline hydroxylation. Am J Respir Cell Mol Biol. 2018;58(3):378–90. https://doi.org/10.1165/rcmb.2017-0238OC.
Cui H, Xie N, Jiang D, et al. Inhibition of glutaminase 1 attenuates experimental pulmonary fibrosis. Am J Respir Cell Mol Biol. 2019;61(4):492–500. https://doi.org/10.1165/rcmb.2019-0051OC.
Zi Z. Molecular engineering of the TGF-β signaling pathway. J Mol Biol. 2019;431(15):2644–54. https://doi.org/10.1016/j.jmb.2019.05.022.
Marrone G, Shah VH, Gracia-Sancho J. Sinusoidal communication in liver fibrosis and regeneration. J Hepatol. 2016;65(3):608–17. https://doi.org/10.1016/j.jhep.2016.04.018.
Meyer A, Wang W, Qu J, et al. Platelet TGF-β1 contributions to plasma TGF-β1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overload. Blood. 2012;119(4):1064–74. https://doi.org/10.1182/blood-2011-09-377648.
Celada LJ, Kropski JA, Herazo-Maya JD, et al. PD-1 up-regulation on CD4(+) T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production. Science translational medicine. 2018;10(460). https://doi.org/10.1126/scitranslmed.aar8356
Nevers T, Salvador AM, Velazquez F, et al. Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. J Exp Med. 2017;214(11):3311–29. https://doi.org/10.1084/jem.20161791.
Gordon JR, Galli SJ. Promotion of mouse fibroblast collagen gene expression by mast cells stimulated via the Fc epsilon RI. Role for mast cell-derived transforming growth factor beta and tumor necrosis factor alpha. The Journal of Experimental Medicine. 1994;180(6):2027–37. https://doi.org/10.1084/jem.180.6.2027.
Young LR, Gulleman PM, Short CW, et al. Epithelial-macrophage interactions determine pulmonary fibrosis susceptibility in Hermansky-Pudlak syndrome. JCI insight. 2016;1(17): e88947. https://doi.org/10.1172/jci.insight.88947.
Juban G, Saclier M, Yacoub-Youssef H, et al. AMPK activation regulates LTBP4-dependent TGF-β1 secretion by pro-inflammatory macrophages and controls fibrosis in Duchenne muscular dystrophy. Cell Rep. 2018;25(8):2163-76.e6. https://doi.org/10.1016/j.celrep.2018.10.077.
Klinkhammer BM, Floege J, Boor P. PDGF in organ fibrosis. Mol Aspects Med. 2018;62:44–62. https://doi.org/10.1016/j.mam.2017.11.008.
Shah M, Foreman DM, Ferguson MW. Neutralising antibody to TGF-beta 1,2 reduces cutaneous scarring in adult rodents. J Cell Sci. 1994;107(Pt 5):1137–57.
Cordeiro MF, Mead A, Ali RR, et al. Novel antisense oligonucleotides targeting TGF-beta inhibit in vivo scarring and improve surgical outcome. Gene Ther. 2003;10(1):59–71. https://doi.org/10.1038/sj.gt.3301865.
Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106(11):1675–80. https://doi.org/10.1161/circresaha.110.217737.
Martin CJ, Datta A, Littlefield C, et al. Selective inhibition of TGFβ1 activation overcomes primary resistance to checkpoint blockade therapy by altering tumor immune landscape. Science translational medicine. 2020;12(536). https://doi.org/10.1126/scitranslmed.aay8456
de Streel G, Bertrand C, Chalon N, et al. Selective inhibition of TGF-β1 produced by GARP-expressing Tregs overcomes resistance to PD-1/PD-L1 blockade in cancer. Nat Commun. 2020;11(1):4545. https://doi.org/10.1038/s41467-020-17811-3.
Cully M. TGFβ1-specific antibody spurs anti-tumour immunity. Nat Rev Drug Discovery. 2020;19(5):310. https://doi.org/10.1038/d41573-020-00058-4.
Tremblay D, Mascarenhas J. Next Generation therapeutics for the treatment of myelofibrosis. Cells. 2021;10(5). https://doi.org/10.3390/cells10051034
Varricchio L, Iancu-Rubin C, Upadhyaya B, et al. TGF-β1 protein trap AVID200 beneficially affects hematopoiesis and bone marrow fibrosis in myelofibrosis. JCI insight. 2021;6(18). https://doi.org/10.1172/jci.insight.145651
Kim BG, Malek E, Choi SH, et al. Novel therapies emerging in oncology to target the TGF-β pathway. J Hematol Oncol. 2021;14(1):55. https://doi.org/10.1186/s13045-021-01053-x.
Ding Y, Xiao L, Chen R, et al. Efficacy and safety of inhaled anti-fibrotic peptide HTPEP-001 in rat models. A64 THERAPEUTICS, DRUG DELIVERY, AND TISSUE ENGINEERING. p. A2286-A. https://doi.org/10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A2286
Roy A, Bera S. CAF cellular glycolysis: linking cancer cells with the microenvironment. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2016;37(7):8503–14. https://doi.org/10.1007/s13277-016-5049-3.
Walshe TE. TGF-beta and microvessel homeostasis. Microvasc Res. 2010;80(1):166–73. https://doi.org/10.1016/j.mvr.2010.03.003.
Morikawa M, Derynck R, Miyazono K. TGF-β and the TGF-β family: context-dependent roles in cell and tissue physiology. Cold Spring Harbor perspectives in biology. 2016;8(5). https://doi.org/10.1101/cshperspect.a021873
Dewidar B, Meyer C, Dooley S, et al. TGF-β in hepatic stellate cell activation and liver fibrogenesis-updated 2019. Cells. 2019;8(11). https://doi.org/10.3390/cells8111419
Sathiyamoorthy G, Sehgal S, Ashton RW. Pirfenidone and nintedanib for treatment of idiopathic pulmonary fibrosis. Southern Med J. 2017;110(6):393–398. https://doi.org/10.14423/smj.0000000000000655
Lan Y, Zhang D, Xu C, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci Translat Med. 2018;10(424). https://doi.org/10.1126/scitranslmed.aan5488
Strauss J, Heery CR, Schlom J, et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFβ, in advanced solid tumors. Clinical Cancer Research. 2018;24(6):1287–95. https://doi.org/10.1158/1078-0432.Ccr-17-2653.
Knudson KM, Hicks KC, Luo X, et al. M7824, a novel bifunctional anti-PD-L1/TGFβ trap fusion protein, promotes anti-tumor efficacy as monotherapy and in combination with vaccine. Oncoimmunology. 2018;7(5): e1426519. https://doi.org/10.1080/2162402x.2018.1426519.
Acknowledgements
We acknowledge the National Natural Science Foundation of China (Grant No. 81773179, 82071399, 21976210), Provincial Natural Science Foundation of Hunan (2020JJ4771), Incubation Project of Medical Science and Technology Youth Training Program (Item Number: 20QNPY143), and Independent Exploration and Innovation Project for Postgraduate of Central South University (Item Number: 2021zzts0929) for providing financial support for this research.
Author information
Authors and Affiliations
Contributions
LLL, RCP, and ZY conceived and designed the study. SN and WZH wrote the paper. ZHC, LWD, ZM, JXJ, and ZJ critically reviewed and revised the manuscript. All authors read and approved the manuscript. SN and WZH contributed equally to this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, N., Wang, Z., Zhu, H. et al. Research progress on drugs targeting the TGF-β signaling pathway in fibrotic diseases. Immunol Res 70, 276–288 (2022). https://doi.org/10.1007/s12026-022-09267-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-022-09267-y