Abstract
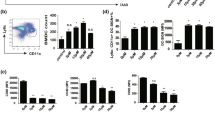
Regulatory dendritic cells are a potential therapeutic tool for assessing a variety of immune overreaction diseases. Paeoniflorin, a bioactive glucoside extracted from the Chinese herb white paeony root, has been shown to be effective at inhibiting the maturation and immunostimulatory function of murine bone marrow-derived dendritic cells. However, whether paeoniflorin can program conventional dendritic cells toward regulatory dendritic cells and the underlying mechanism remain unknown. Here, our study demonstrates that paeoniflorin can induce the production of regulatory dendritic cells from human peripheral blood monocyte-derived immature dendritic cells in the absence or presence of lipopolysaccharide (LPS) but not from mature dendritic cells, thereby demonstrating the potential of paeoniflorin as a specific immunosuppressive drug with fewer complications and side effects. These regulatory dendritic cells treated with paeoniflorin exhibited high CD11b/c and low CD80, CD86 and CD40 expression levels as well as enhanced abilities to capture antigen and promote the proliferation of CD4+CD25+ T cells and reduced abilities to migrate and promote the proliferation of CD4+ T cells, which is associated with the upregulation of endogenous transforming growth factor (TGF)-β-mediated indoleamine 2,3-dioxygenase (IDO) expression. Collectively, paeoniflorin could program immature dendritic cells (imDCs) and imDCs stimulated with LPS toward a regulatory DC fate by upregulating the endogenous TGF-β-mediated IDO expression level, thereby demonstrating its potential as a specific immunosuppressive drug.








Similar content being viewed by others
Abbreviations
- ANOVA:
-
One-way analysis of variance
- CD:
-
Cluster of differentiation
- DCs:
-
Dendritic cells
- DCregs:
-
Regulatory dendritic cells
- FCS:
-
Fetal calf serum
- FITC:
-
Fluorescein isothiocyanate
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- HLA:
-
Human leukocyte antigen
- HRP:
-
Horseradish peroxidase
- IL:
-
Interleukin
- imDCs:
-
Immature dendritic cells
- IDO:
-
Indoleamine 2,3-dioxygenase
- LPS:
-
Lipopolysaccharide
- mDCs:
-
Mature dendritic cells
- PBMCs:
-
Peripheral blood mononuclear cells
- PBS:
-
Phosphate-buffered saline
- PI:
-
Propidium iodide
- TGF:
-
Transforming growth factor
- Tregs:
-
Regulatory T cells
- Pae:
-
Paeoniflorin
- 1-MT:
-
1-Methytryptophan
References
Han Y, Chen Z, Yang Y, Jiang Z, Gu Y, Liu Y, et al. Human CD14 + CTLA-4 + regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology. 2014;59(2):567–79. doi:10.1002/hep.26694.
Ezzelarab M, Thomson AW. Tolerogenic dendritic cells and their role in transplantation. Semin Immunol. 2011;23(4):252–63. doi:10.1016/j.smim.2011.06.007.
Harimoto H, Shimizu M, Nakagawa Y, Nakatsuka K, Wakabayashi A, Sakamoto C, et al. Inactivation of tumor-specific CD8(+) CTLs by tumor-infiltrating tolerogenic dendritic cells. Immunol Cell Biol. 2013;91(9):545–55. doi:10.1038/icb.2013.38.
Raich-Regue D, Glancy M, Thomson AW. Regulatory dendritic cell therapy: from rodents to clinical application. Immunol Lett. 2014;161(2):216–21. doi:10.1016/j.imlet.2013.11.016.
Li H, Shi B. Tolerogenic dendritic cells and their applications in transplantation. Cell Mol Immunol. 2015;12(1):24–30. doi:10.1038/cmi.2014.52.
Schmidt SV, Nino-Castro AC, Schultze JL. Regulatory dendritic cells: there is more than just immune activation. Front Immunol. 2012;3:274. doi:10.3389/fimmu.2012.00274.
Liu Q, Zhang C, Sun A, Zheng Y, Wang L, Cao X. Tumor-educated CD11bhighIalow regulatory dendritic cells suppress T cell response through arginase I. J Immunol. 2009;182(10):6207–16. doi:10.4049/jimmunol.0803926.
Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4(1):24–34. doi:10.1038/nri1256.
Taher YA, van Esch BC, Hofman GA, Henricks PA, van Oosterhout AJ. 1alpha,25-dihydroxyvitamin D3 potentiates the beneficial effects of allergen immunotherapy in a mouse model of allergic asthma: role for IL-10 and TGF-beta. J Immunol. 2008;180(8):5211–21.
Owens BM, Kaye PM. Stromal cell induction of regulatory dendritic cells. Front Immunol. 2012;3:262. doi:10.3389/fimmu.2012.00262.
Li G, Abediankenari S, Kim YJ, Campbell TB, Ito S, Graham-Evans B, et al. TGF-beta combined with M-CSF and IL-4 induces generation of immune inhibitory cord blood dendritic cells capable of enhancing cytokine-induced ex vivo expansion of myeloid progenitors. Blood. 2007;110(8):2872–9. doi:10.1182/blood-2006-10-050583.
Torres-Aguilar H, Aguilar-Ruiz SR, Gonzalez-Perez G, Munguia R, Bajana S, Meraz-Rios MA, et al. Tolerogenic dendritic cells generated with different immunosuppressive cytokines induce antigen-specific anergy and regulatory properties in memory CD4 + T cells. J Immunol. 2010;184(4):1765–75. doi:10.4049/jimmunol.0902133.
Cai Z, Zhang W, Li M, Yue Y, Yang F, Yu L, et al. TGF-beta1 gene-modified, immature dendritic cells delay the development of inflammatory bowel disease by inducing CD4(+)Foxp3(+) regulatory T cells. Cell Mol Immunol. 2010;7(1):35–43. doi:10.1038/cmi.2009.107.
Zheng X, Suzuki M, Ichim TE, Zhang X, Sun H, Zhu F, et al. Treatment of autoimmune arthritis using RNA interference-modulated dendritic cells. J Immunol. 2010;184(11):6457–64. doi:10.4049/jimmunol.0901717.
Campos-Acuna J, Perez F, Narvaez E, Campos-Mora M, Gajardo T, Catalan D, et al. Rapamycin-conditioned dendritic cells activated with monophosphoryl lipid-A promote allograft acceptance in vivo. Immunotherapy. 2015;7(2):101–10. doi:10.2217/imt.14.116.
Macedo C, Turquist H, Metes D, Thomson AW. Immunoregulatory properties of rapamycin-conditioned monocyte-derived dendritic cells and their role in transplantation. Transplant Res. 2012;1(1):16. doi:10.1186/2047-1440-1-16.
de Freitas KM, Almeida JM, Monteiro JC, Diamante MA, do Vale JS, Camargo C, et al. The effects of cyclosporin A and Heteropterys tomentosa on the rat liver. Anais da Academia Brasileira de Ciencias. 2015;87(1):369–79. doi:10.1590/0001-3765201520130351.
He DY, Dai SM. Anti-inflammatory and immunomodulatory effects of paeonia lactiflora pall., a traditional chinese herbal medicine. Front Pharmacol. 2011;2:10. doi:10.3389/fphar.2011.00010.
Feng L, Liu WK, Deng L, Tian JX, Tong XL. Clinical efficacy of aconitum-containing traditional Chinese medicine for diabetic peripheral neuropathic pain. Am J Chin Med. 2014;42(1):109–17. doi:10.1142/S0192415X14500074.
Xu W, Zhou L, Ma X, Chen Y, Qin B, Zhai X, et al. Therapeutic effects of combination of paeoniflorin and albiflorin from Paeonia radix on radiation and chemotherapy-induced myelosuppression in mice and rabbits. Asian Pac J Cancer Prev APJCP. 2011;12(8):2031–7.
Wang C, Yuan J, Wu HX, Chang Y, Wang QT, Wu YJ, et al. Paeoniflorin inhibits inflammatory responses in mice with allergic contact dermatitis by regulating the balance between inflammatory and anti-inflammatory cytokines. Inflamm Res Off J Eur Histamine Res Soc. 2013;62(12):1035–44. doi:10.1007/s00011-013-0662-8.
Wu H, Wei W, Song L, Zhang L, Chen Y, Hu X. Paeoniflorin induced immune tolerance of mesenteric lymph node lymphocytes via enhancing beta 2-adrenergic receptor desensitization in rats with adjuvant arthritis. Int Immunopharmacol. 2007;7(5):662–73. doi:10.1016/j.intimp.2007.01.019.
Li PP, Liu DD, Liu YJ, Song SS, Wang QT, Chang Y, et al. BAFF/BAFF-R involved in antibodies production of rats with collagen-induced arthritis via PI3 K-Akt-mTOR signaling and the regulation of paeoniflorin. J Ethnopharmacol. 2012;141(1):290–300. doi:10.1016/j.jep.2012.02.034.
Nam KN, Yae CG, Hong JW, Cho DH, Lee JH, Lee EH. Paeoniflorin, a monoterpene glycoside, attenuates lipopolysaccharide-induced neuronal injury and brain microglial inflammatory response. Biotechnol Lett. 2013;35(8):1183–9. doi:10.1007/s10529-013-1192-8.
Shi D, Li X, Li D, Zhao Q, Shen Y, Yan H, et al. Oral administration of paeoniflorin attenuates allergic contact dermatitis by inhibiting dendritic cell migration and Th1 and Th17 differentiation in a mouse model. Int Immunopharmacol. 2015;25(2):432–9. doi:10.1016/j.intimp.2015.02.031.
Shi D, Ma A, Zheng H, Huo G, Yan H, Fu H, et al. Paeoniflorin inhibits the maturation and immunostimulatory function of allergen-induced murine dendritic cells. Int Immunopharmacol. 2014;19(2):221–32. doi:10.1016/j.intimp.2014.02.001.
Xiong A, Duan L, Chen J, Fan Z, Zheng F, Tan Z, et al. Flt3L combined with rapamycin promotes cardiac allograft tolerance by inducing regulatory dendritic cells and allograft autophagy in mice. PLoS One. 2012;7(10):e46230. doi:10.1371/journal.pone.0046230.
Lan YY, Wang Z, Raimondi G, Wu W, Colvin BL, de Creus A, et al. “Alternatively activated” dendritic cells preferentially secrete IL-10, expand Foxp3 + CD4 + T cells, and induce long-term organ allograft survival in combination with CTLA4-Ig. J Immunol. 2006;177(9):5868–77.
Hilkens CM, Isaacs JD. Tolerogenic dendritic cell therapy for rheumatoid arthritis: Where are we now? Clin Exp Immunol. 2013;172(2):148–57. doi:10.1111/cei.12038.
Nadig SN, Wieckiewicz J, Wu DC, Warnecke G, Zhang W, Luo S, et al. In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med. 2010;16(7):809–13. doi:10.1038/nm.2154.
Tang Q, Bluestone JA, Kang SM. CD4(+)Foxp3(+) regulatory T cell therapy in transplantation. J Mol Cell Biol. 2012;4(1):11–21. doi:10.1093/jmcb/mjr047.
Laouar Y, Town T, Jeng D, Tran E, Wan Y, Kuchroo VK, et al. TGF-beta signaling in dendritic cells is a prerequisite for the control of autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2008;105(31):10865–70. doi:10.1073/pnas.0805058105.
Garate D, Rojas-Colonelli N, Pena C, Salazar L, Abello P, Pesce B, et al. Blocking of p38 and transforming growth factor beta receptor pathways impairs the ability of tolerogenic dendritic cells to suppress murine arthritis. Arthr Rheum. 2013;65(1):120–9. doi:10.1002/art.37702.
Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206–21. doi:10.1111/j.1600-065X.2008.00610.x.
Yu G, Fang M, Gong M, Liu L, Zhong J, Feng W, et al. Steady state dendritic cells with forced IDO expression induce skin allograft tolerance by upregulation of regulatory T cells. Transpl Immunol. 2008;18(3):208–19. doi:10.1016/j.trim.2007.08.006.
Cook CH, Bickerstaff AA, Wang JJ, Nadasdy T, Della Pelle P, Colvin RB, et al. Spontaneous renal allograft acceptance associated with “regulatory” dendritic cells and IDO. J Immunol. 2008;180(5):3103–12.
Yang J, Yang Y, Fan H, Zou H. Tolerogenic splenic IDO (+) dendritic cells from the mice treated with induced-Treg cells suppress collagen-induced arthritis. J Immunol Res. 2014;2014:831054. doi:10.1155/2014/831054.
An XJ, Bai CX, Xia JB, Dang T, Qian P, Qian GS, et al. Immature dendritic cells expressing indoleamine 2,3-dioxygenase suppress ovalbumin-induced allergic airway inflammation in mice. J Investig Allergol Clin Immunol. 2011;21(3):185–92.
Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12(9):870–8. doi:10.1038/ni.2077.
Fallarino F, Grohmann U, Puccetti P. Indoleamine 2,3-dioxygenase: from catalyst to signaling function. Eur J Immunol. 2012;42(8):1932–7. doi:10.1002/eji.201242572.
Song SS, Yuan PF, Chen JY, Fu JJ, Wu HX, Lu JT, et al. TGF-beta favors bone marrow-derived dendritic cells to acquire tolerogenic properties. Immunol Invest. 2014;43(4):360–9. doi:10.3109/08820139.2013.879172.
Belladonna ML, Volpi C, Bianchi R, Vacca C, Orabona C, Pallotta MT, et al. Cutting edge: Autocrine TGF-beta sustains default tolerogenesis by IDO-competent dendritic cells. J Immunol. 2008;181(8):5194–8.
Acknowledgments
We greatly acknowledge the technical assistance from Professor Feng Wang and Yi Liu. We are also grateful to teacher Zhiyan Zhu for the assistant in the FAC sorting and analysis. This work was supported by National High Technology Development Project (2012AA021003), National Natural Science Foundation of China (21177091) and Tianjin Science and Technology Support Program (12ZCZDSY03400).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
This manuscript does not contain any financial/commercial conflicts of interests. The human participants provided informed consent for the experimental study, which has been reviewed and approved by the ethics committee of Tianjin Medical University and in accordance with the 1964 Helsinki Declaration.
Conflict of interest
The authors declare no financial or commercial conflicts of interest.
Rights and permissions
About this article
Cite this article
Chen, D., Li, Y., Wang, X. et al. Generation of regulatory dendritic cells after treatment with paeoniflorin. Immunol Res 64, 988–1000 (2016). https://doi.org/10.1007/s12026-015-8773-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-015-8773-7