Abstract
Amphicrine carcinomas are epithelial neoplasms composed of cells with co-existing exocrine-neuroendocrine phenotype and are challenging lesions from both diagnostic and therapeutic perspectives.
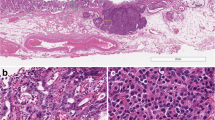
Here, we report the case of a 63-year-old male patient with a gastric nodule that was endoscopically biopsied, revealing histological features of a type 3 well-differentiated gastric neuroendocrine tumor (NET). At imaging, the lesion was single and limited to the stomach, but did not present In−111Octreotide uptake, despite SSTR2A immunohistochemical expression. The patient underwent a wedge resection of the gastric wall, with a final pathological diagnosis of amphicrine carcinoma with pancreatic acinar cell and neuroendocrine features (pT1b). Predictive immunohistochemistry showed microsatellite stability and negative HER2 status. Hotspot targeted deep sequencing of 57 genes showed no somatic mutation, in agreement with the low mutational burden reported for gastric amphicrine carcinomas. Due to a low stage of the tumor and the poor performance status of the patient, no additional oncological treatment was administered. The patient was disease-free after 18 months.
This unusual case highlights the importance of considering amphicrine carcinoma in the diagnostic work-up of gastric type 3 NET. This can be done by including in the immunohistochemical panel non-neuroendocrine markers, such as the pancreatic acinar cell and glandular ones. Correct pathological diagnosis is pivotal to determine the appropriate staging (NET vs exocrine one) for surgical and oncological management.




Similar content being viewed by others
References
Lewin K Carcinoid tumors and the mixed (composite) glandular-endocrine cell carcinomas. Am J Surg Pathol 11 Suppl. 1: 71–86, 1987.
Rindi G, Mete O, Uccella S, et al. Overview of the 2022 WHO classification of neuroendocrine neoplasms. Endocr Pathol 33: 115-154, 2022.
Bellur S, Van der Kwast T, Mete O. Evolving concepts in prostatic neuroendocrine manifestations: from focal divergent differentiation to amphicrine carcinoma. Hum Pathol 85: 313–327, 2019.
Khandakar H, Agarwal S, Sharma MC, et al. Amphicrine medullary thyroid carcinoma - a case-based review expanding on its MUC expression profile. Endocr Pathol 33: 378–387, 2022.
Ganesan K, Achmad E, Sirlin CB, et al. Amphicrine carcinoma of the liver. Ann Diagn Pathol 15: 355–357, 2011.
Taggart MW, Abraham SC, Overman MJ, Mansfield PF, Rashid A. Goblet cell carcinoid tumor, mixed goblet cell carcinoid-adenocarcinoma, and adenocarcinoma of the appendix: comparison of clinicopathologic features and prognosis. Arch Pathol Lab Med 139: 782–790, 2015.
Ginori A, Lo Bello G, Vassallo L, Tripodi SA. Amphicrine carcinoma of the ampullary region. Tumori 101: e70–72, 2015.
Brouland JP, Manivet P, Brocheriou-Spelle I, et al. Histological, immunohistochemical, ultrastructural and biochemical study of human gastric composite tumor: expression of the serotonin-2B receptor by the neuroendocrine component. Endocr Pathol 12: 77–86, 2001.
Osamura RY GA, Tallini G, Volante M, Bongiovanni M, Fuchs TL. Medullary thyroid carcinoma. In: WHO Classification of Tumours Editorial Board. Endocrine and neuroendocrine tumours. Lyon (France): International Agency for Research on Cancer; 2022. (WHO classification of tumours series, 5th ed.; vol. 10). https://publications.iarc.fr.
Misdraji J, Carr NJ, Pai RK. Appendiceal goblet cell adenocarcinoma. In: WHO classification of tumours editorial board. Digestive system tumours. Lyon (France): International Agency for Research on Cancer; 2019. (WHO classification of tumours series, 5th ed.; vol. 1). https://publications.iarc.fr/579.
Palmer K, Weerasuriya S, Chandrakumaran K, et al. Goblet cell adenocarcinoma of the appendix: a systematic review and incidence and survival of 1,225 cases from an English Cancer Registry. Front Oncol 12: 915028, 2022.
Huang D, Ren F, Ni S, et al. Amphicrine carcinoma of the stomach and intestine: a clinicopathologic and pan-cancer transcriptome analysis of a distinct entity. Cancer Cell Int 19: 310, 2019.
Sun L, Wang C, Zhang Jet al. Genetic alterations in gastric amphicrine carcinomas and comparison with gastric mixed neuroendocrine-non-neuroendocrine neoplasms. Mod Pathol 35: 808-815, 2022.
Fukunaga M. Gastric carcinoma resembling pancreatic mixed acinar-endocrine carcinoma. Hum Pathol 33: 569–573, 2002.
La Rosa S, Sessa F, Uccella S. Mixed neuroendocrine-nonneuroendocrine neoplasms (MiNENs): unifying the concept of a heterogeneous group of neoplasms. Endocr Pathol 27: 284–311, 2016.
Yozu M, Johncilla ME, Srivastava A, et al. Histologic and outcome study supports reclassifying appendiceal goblet cell carcinoids as goblet cell adenocarcinomas, and grading and staging similarly to colonic adenocarcinomas. Am J Surg Pathol 42: 898-910, 2018.
Jain D, Eslami-Varzaneh F, Takano AM, et al. Composite glandular and endocrine tumors of the stomach with pancreatic acinar differentiation. Am J Surg Pathol 29: 1524–1529, 2005.
Mastracci L, Rindi G, Grillo F, et al. Neuroendocrine neoplasms of the esophagus and stomach. Pathologica 113: 5-11, 2021.
de Mestier L, Cros J, Neuzillet C, et al. Digestive system mixed neuroendocrine-non-neuroendocrine neoplasms. Neuroendocrinology 105: 412-425, 2017.
Fujita Y, Uesugi N, Sugimoto R, Eizuka M, Matsumoto T, Sugai T. Gastric mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN) with pancreatic acinar differentiation: a case report. Diagn Pathol 14: 38, 2019.
Ooe Y, Watanabe K, Hashimoto I, et al. Pancreatic-type mixed acinar neuroendocrine carcinoma of the stomach: a case report and review of the literature. Diagn Pathol 16: 11, 2021.
Yang GC, Rotterdam H. Mixed (composite) glandular-endocrine cell carcinoma of the stomach. Report of a case and review of literature. Am J Surg Pathol 15: 592–598, 1991.
Chejfec G, Capella C, Solcia E, Jao W, Gould VE. Amphicrine cells, dysplasias, and neoplasias. Cancer 56: 2683–2690, 1985.
Volante M, Brizzi MP, Faggiano A, et al. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod Pathol 20: 1172–1182, 2007.
La Rosa S, Sessa F, Capella C. Acinar cell carcinoma of the pancreas: overview of clinicopathologic features and insights into the molecular pathology. Front Med (Lausanne) 2: 41, 2015.
Sarkar M, Lai JC, Sawinski D, et al. Sex hormone levels by presence and severity of cirrhosis in women with chronic hepatitis C virus (HCV) infection. J Viral Hepat 26: 258–262, 2019.
Uccella S, La Rosa S. Looking into digestive mixed neuroendocrine - nonneuroendocrine neoplasms: subtypes prognosis, and predictive factors. Histopathology 5: 700–717, 2020.
Author information
Authors and Affiliations
Contributions
AS, SLR study design, data collection, drafting. SU, PH, IF, VS, GF data collection, critical review of the manuscript. All authors approved the final version of the paper.
Corresponding author
Ethics declarations
Ethics Approval
Informed consent was obtained from the patient. Ethic committee approval: not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sciarra, A., Uccella, S., Hiroz, P. et al. Gastric Amphicrine Carcinoma Showing Neuroendocrine and Pancreatic Acinar Cell Differentiation. Lesson from a Challenging Case Opening New Perspectives in the Diagnostic Work-Up of Gastric Neuroendocrine Neoplasms. Endocr Pathol 34, 349–357 (2023). https://doi.org/10.1007/s12022-023-09773-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-023-09773-1