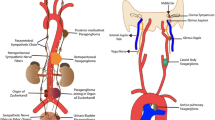
Abstract
This review summarizes the classification of tumors of the adrenal medulla and extra-adrenal paraganglia as outlined in the 5th series of the WHO Classification of Endocrine and Neuroendocrine Tumors. The non-epithelial neuroendocrine neoplasms (NENs) known as paragangliomas produce predominantly catecholamines and secrete them into the bloodstream like hormones, and they represent a group of NENs that have exceptionally high genetic predisposition. This classification discusses the embryologic derivation of the cells that give rise to these lesions and the historical evolution of the terminology used to classify their tumors; paragangliomas can be sympathetic or parasympathetic and the term pheochromocytoma is used specifically for intra-adrenal paragangliomas that represent the classical sympathetic form. In addition to the general neuroendocrine cell biomarkers INSM1, synaptophysin, and chromogranins, these tumors are typically negative for keratins and instead have highly specific biomarkers, including the GATA3 transcription factor and enzymes involved in catecholamine biosynthesis: tyrosine hydroxylase that converts L-tyrosine to L-DOPA as the rate-limiting step in catecholamine biosynthesis, dopamine beta-hydroxylase that is present in cells expressing norepinephrine, and phenylethanolamine N-methyltransferase, which converts norepinephrine to epinephrine and therefore can be used to distinguish tumors that make epinephrine. In addition to these important tools that can be used to confirm the diagnosis of a paraganglioma, new tools are recommended to determine genetic predisposition syndromes; in addition to the identification of precursor lesions, molecular immunohistochemistry can serve to identify associations with SDHx, VHL, FH, MAX, and MEN1 mutations, as well as pseudohypoxia-related pathogenesis. Paragangliomas have a well-formed network of sustentacular cells that express SOX10 and S100, but this is not a distinctive feature, as other epithelial NENs also have sustentacular cells. Indeed, it is the presence of such cells and the association with ganglion cells that led to a misinterpretation of several unusual lesions as paragangliomas; in the 2022 WHO classification, the tumor formerly known as cauda equina paraganglioma is now classified as cauda equina neuroendocrine tumor and the lesion known as gangliocytic paraganglioma has been renamed composite gangliocytoma/neuroma and neuroendocrine tumor (CoGNET). Since the 4th edition of the WHO, paragangliomas have no longer been classified as benign and malignant, as any lesion can have metastatic potential and there are no clear-cut features that can predict metastatic behavior. Moreover, some tumors are lethal without metastatic spread, by nature of local invasion involving critical structures. Nevertheless, there are features that can be used to identify more aggressive lesions; the WHO does not endorse the various scoring systems that are reviewed but also does not discourage their use. The identification of metastases is also complex, particularly in patients with germline predisposition syndromes, since multiple lesions may represent multifocal primary tumors rather than metastatic spread; the identification of paragangliomas in unusual locations such as lung or liver is not diagnostic of metastasis, since these may be primary sites. The value of sustentacular cells and Ki67 labeling as prognostic features is also discussed in this new classification. A staging system for pheochromocytoma and extra-adrenal sympathetic PGLs, introduced in the 8th Edition AJCC Cancer Staging Manual, is now included. This paper also provides a summary of the criteria for the diagnosis of a composite paragangliomas and summarizes the classification of neuroblastic tumors. This review adopts a practical question–answer framework to provide members of the multidisciplinary endocrine oncology team with a most up-to-date approach to tumors of the adrenal medulla and extra-adrenal paraganglia.






















Similar content being viewed by others
References
Carmichael SW, Rochester. The history of the adrenal medulla. Rev Neurosci 1989;2:83–100.
Kohn A: Die chromaffinen Zellen des sympathicus. Anat Anz 1898; 15:399-400.
Kohn A: Die Paraganglien. Arch Mikr Anat 1903; 52:262-365.
Zak FG, Lawson. The Paraganglionic Chemoreceptor System. Physiology, Pathology and Clinical Medicine. New York: Springer-Verlag, 1982.
Kastriti ME, Kameneva P, Kamenev D, Dyachuk V, Furlan A, Hampl M, Memic F, Marklund U, Lallemend F, Hadjab S, Calvo-Enrique L, Ernfors P, Fried K, Adameyko I. Schwann Cell Precursors Generate the Majority of Chromaffin Cells in Zuckerkandl Organ and Some Sympathetic Neurons in Paraganglia. Front Mol Neurosci. 2019;12:6.
Furlan A, Adameyko I: Schwann cell precursor: a neural crest cell in disguise? Dev Biol 2018; 444 Suppl 1:S25-S35.
Furlan A, Dyachuk V, Kastriti ME, Calvo-Enrique L, Abdo H, Hadjab S, Chontorotzea T, Akkuratova N, Usoskin D, Kamenev D, Petersen J, Sunadome K, Memic F, Marklund U, Fried K, Topilko P, Lallemend F, Kharchenko PV, Ernfors P, Adameyko I. Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science. 2017;357(6346):eaal3753.
Hockman D, Adameyko I, Kaucka M, Barraud P, Otani T, Hunt A, Hartwig AC, Sock E, Waithe D, Franck MCM, Ernfors P, Ehinger S, Howard MJ, Brown N, Reese J, Baker CVH. Striking parallels between carotid body glomus cell and adrenal chromaffin cell development. Dev Biol. 2018;444 Suppl 1(Suppl 1):S308-S324.
Kameneva P, Artemov AV, Kastriti ME, Faure L, Olsen TK, Otte J, Erickson A, Semsch B, Andersson ER, Ratz M, Frisén J, Tischler AS, de Krijger RR, Bouderlique T, Akkuratova N, Vorontsova M, Gusev O, Fried K, Sundström E, Mei S, Kogner P, Baryawno N, Kharchenko PV, Adameyko I. Single-cell transcriptomics of human embryos identifies multiple sympathoblast lineages with potential implications for neuroblastoma origin. Nat Genet. 2021;53(5):694-706.
Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev 1998; 12:3320-4.
Fraenkel F. A case of bilateral, completely latent adrenal tumor and concurrent nephritis with changes in the circulatory system and retinitis. CA- A cancer journal for clinicians 1984; 34:93-106 (translated from original German version 1884).
Bausch B, Tischler AS, Schmid KW, Leijon H, Eng C, Neumann HPH. Max Schottelius: Pioneer in Pheochromocytoma. J Endocr Soc 2017; 1:957-964.
Pick L. Das Ganglioma embryonale sympathicum (Sympathoma embryonale), eine typische bosartige geschwuestform des sympathischen nervensystems. Berl Klin Wochenschr 1912; 49:16-22.
Karsner HT. Tumors of the Adrenal. Atlas of Tumor Pathology, Section VIII-Fascicle 29. Washington, DC: Armed Forces Institute of Pathology, 1950
Pacak K, Eisenhofer G, Tischler AS. Phaeochromocytoma-advances through science, collaboration and spreading the word. Nat Rev Endocrinol 2020; 16:621-622.
16 Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, Busam KJ, de Krijger RR, Dietel M, El-Naggar AK, Fernandez-Cuesta L, Klöppel G, McCluggage WG, Moch H, Ohgaki H, Rakha EA, Reed NS, Rous BA, Sasano H, Scarpa A, Scoazec JY, Travis WD, Tallini G, Trouillas J, van Krieken JH, Cree IA. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol 2018;31:1770-86.
Moriguchi T, Takako N, Hamada M et al. Gata3 participates in a complex transcriptional feedback network to regulate sympathoadrenal differentiation. Development 2006;133:3871-81.
Uccella S, Asa SL, Mete O. Neuroendocrine neoplasms of unknown primary site. In: Asa SL, La Rosa S, Mete O, editors. The Spectrum of Neuroendocrine Neoplasia. Springer; 2020.
Asa SL, Lloyd RV, Tischler AS. Neuroendocrine neoplasms: Historical background and terminologies. In: Asa SL, La Rosa S, Mete O, editors. The Spectrum of Neuroendocrine Neoplasia. Springer; 2020.
Mete O, Essa A, Bramdev A, Govender N, Chetty R. MEN2 Syndrome-Related Medullary Thyroid Carcinoma with Focal Tyrosine Hydroxylase Expression: Does It Represent a Hybrid Cellular Phenotype or Functional State of Tumor Cells? Endocr Pathol. 2017;28:362-366.
Mamilla D, Manukyan I, Fetsch PA, Pacak K, Miettinen M. Immunohistochemical distinction of paragangliomas from epithelial neuroendocrine tumors-gangliocytic duodenal and cauda equina paragangliomas align with epithelial neuroendocrine tumors. Hum Pathol. 2020;103:72-82.
Ramani B, Gupta R, Wu J, Barreto J, Bollen AW, Tihan T, Mummaneni PV, Ames C, Clark A, Oberheim Bush NA, Butowski N, Phillips D, King BE, Bator SM, Treynor EC, Zherebitskiy V, Quinn PS, Walker JB, Pekmezci M, Sullivan DV, Hofmann JW, Sloan EA, M Chang S, Berger MS, Solomon DA, Perry A. The immunohistochemical, DNA methylation, and chromosomal copy number profile of cauda equina paraganglioma is distinct from extra-spinal paraganglioma. Acta Neuropathol. 2020;140(6):907–917.
Dermawan JK, Mukhopadhyay S, Shah AA. Frequency and extent of cytokeratin expression in paraganglioma: an immunohistochemical study of 60 cases from 5 anatomic sites and review of the literature. Hum Pathol. 2019;93:16-22.
Mete O, Tischler AS, de Krijger R, McNicol AM, Eisenhofer G, Pacak K, Ezzat S, Asa SL. Protocol for the examination of specimens from patients with pheochromocytomas and extra-adrenal paragangliomas. Arch Pathol Lab Med. 2014;138:182-188.
Gucer H, Mete O. Endobronchial gangliocytic paraganglioma: not all keratin-positive endobronchial neuroendocrine neoplasms are pulmonary carcinoids. Endocr Pathol. 2014;25:356-358.
Chetty R. Cytokeratin expression in cauda equina paragangliomas. Am J Surg Pathol. 1999;23:491.
Strommer KN, Brandner S, Sarioglu AC, Sure U, Yonekawa Y. Symptomatic cerebellar metastasis and late local recurrence of a cauda equina paraganglioma. Case report. J Neurosurg. 1995;83:166-169.
Orrell JM, Hales SA. Paragangliomas of the cauda equina have a distinctive cytokeratin immunophenotype. Histopathology. 1992;21:479-481.
So JS, Epstein JI. GATA3 expression in paragangliomas: a pitfall potentially leading to misdiagnosis of urothelial carcinoma. Mod Pathol. 2013;26:1365-1370.
Bockmayr M, Körner M, Schweizer L, Schüller U. Cauda equina paragangliomas express HOXB13. Neuropathol Appl Neurobiol. 2021;47:889-890.
Kaminuma Y, Tanahashi M, Yoshii N, Niwa H. Bronchogenic Gangliocytic Paraganglioma. J Bronchology Interv Pulmonol. 2020;27:212-215.
Nonaka K, Matsuda Y, Okaniwa A, Kasajima A, Sasano H, Arai T. Pancreatic gangliocytic paraganglioma harboring lymph node metastasis: a case report and literature review. Diagn Pathol. 2017;12:57.
Okubo Y, Yokose T, Motohashi O, Miyagi Y, Yoshioka E, Suzuki M, Washimi K, Kawachi K, Nito M, Nemoto T, Shibuya K, Kameda Y. Duodenal Rare Neuroendocrine Tumor: Clinicopathological Characteristics of Patients with Gangliocytic Paraganglioma. Gastroenterol Res Pract. 2016;2016:5257312.
Yang JW, Han J, Lee HW, Cho SY, Kim HK. A Rare Case of Thymic Gangliocytic Paraganglioma. J Pathol Transl Med. 2016;50:165-167.
Witkiewicz A, Galler A, Yeo CJ, Gross SD. Gangliocytic paraganglioma: case report and review of the literature. J Gastrointest Surg. 2007;11:1351-1354.
Sundararajan V, Robinson-Smith TM, Lowy AM. Duodenal gangliocytic paraganglioma with lymph node metastasis: a case report and review of the literature. Arch Pathol Lab Med. 2003;127:e139-41.
Okubo Y. Gangliocytic paraganglioma: An overview and future perspective. World J Clin Oncol. 2019;10:300-302.
Carney JA, Sizemore GW, Sheps SG. Adrenal medullary disease in multiple endocrine neoplasia, type 2: pheochromocytoma and its precursors. Am J Clin Pathol 1976; 66:279-90.
DeLellis RA, Wolfe HJ, Gagel RF, et al.: Adrenal medullary hyperplasia. A morphometric analysis in patients with familial medullary thyroid carcinoma. Am J Pathol 1976; 83:177–96.
Carney JA, Sizemore GW, Tyce GM. Bilateral adrenal medullary hyperplasia in multiple endocrine neoplasia, type 2: the precursor of bilateral pheochromocytoma. Mayo Clin Proc 1975; 50:3-10.
Falhammar H, Stenman A, Calissendorff J, Juhlin CC. Presentation, Treatment, Histology, and Outcomes in Adrenal Medullary Hyperplasia Compared With Pheochromocytoma. Journal of the Endocrine Society 2019; 3:1518-1530.
Neumann HPH, Tsoy U, Bancos I, et al. Comparison of Pheochromocytoma-Specific Morbidity and Mortality Among Adults With Bilateral Pheochromocytomas Undergoing Total Adrenalectomy vs Cortical-Sparing Adrenalectomy. JAMA Network Open 2019;2:e198898-e198898.
Mete O, Asa SL. Precursor lesions of endocrine system neoplasms. Pathology 2013; 45:316-330.
Korpershoek E, Petri BJ, Post E, et al. Adrenal medullary hyperplasia is a precursor lesion for pheochromocytoma in MEN2 syndrome. Neoplasia 2014; 16:868-73.
Romanet P, Guerin C, Pedini P, Essamet W, Castinetti F, Sebag F, Roche P, Cascon A, Tischler AS, Pacak K, Barlier A, Taïeb D. Pathological and Genetic Characterization of Bilateral Adrenomedullary Hyperplasia in a Patient with Germline MAX Mutation. Endocr Pathol. 2017;28:302-307.
Grogan RH, Pacak K, Pasche L, Huynh TT, Greco RS. Bilateral adrenal medullary hyperplasia associated with an SDHB mutation. J Clin Oncol. 2011;29:e200-202.
Hernandez KG, Ezzat S, Morel CF, Swallow C, Otremba M, Dickson BC, Asa SL, Mete O. Familial pheochromocytoma and renal cell carcinoma syndrome: TMEM127 as a novel candidate gene for the association. Virchows Arch. 2015;466:727-732.
Asa SL, Ezzat S, Mete O. The Diagnosis and Clinical Significance of Paragangliomas in Unusual Locations. J Clin Med 2018;7:280.
Kimura N, Ishikawa M, Shigematsu K. Colorectal paragangliomas with immunohistochemical deficiency of succinate dehydrogenase subunit B. Endocrine J. https://doi.org/10.1507/endocrj.EJ21-0 Online ahead of print.
Juhlin CC, Zedenius J, Höög A. Clinical Routine Application of the Second-generation Neuroendocrine Markers ISL1, INSM1, and Secretagogin in Neuroendocrine Neoplasia: Staining Outcomes and Potential Clues for Determining Tumor Origin. Endocr Pathol. 2020;31:401-410.
Duan K, Mete O. Algorithmic approach to neuroendocrine tumors in targeted biopsies: Practical applications of immunohistochemical markers. Cancer Cytopathol. 2016;124:871-884.
Hayashi T, Mete O. Head and Neck Paragangliomas: What does the pathologist need to know? Diagnostic Histopathology. 2014; 20:316-325.
Mete O, Asa SL. Structure, Function, and Morphology in the Classification of Pituitary Neuroendocrine Tumors: the Importance of Routine Analysis of Pituitary Transcription Factors. Endocr Pathol. 2020;31:330-336.
Asa SL, Mete O. Immunohistochemical Biomarkers in Pituitary Pathology. Endocr Pathol. 2018;29:130-136.
Asa SL, Mete O, Perry A, Osamura RY. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr Pathol. 2022. In Press.
Mete O, Pakbaz S, Lerario AM, Giordano TJ, Asa SL. Significance of Alpha-inhibin Expression in Pheochromocytomas and Paragangliomas. Am J Surg Pathol. 2021;45:1264-1273.
Hofland J, van Nederveen FH, Timmerman MA, Korpershoek E, de Herder WW, Lenders JW, Verhofstad AA, de Krijger RR, de Jong FH. Expression of activin and inhibin subunits, receptors and binding proteins in human pheochromocytomas: a study based on mRNA analysis and immunohistochemistry. Clin Endocrinol (Oxf). 2007;66:335-340.
Mete O, Asa SL, Giordano TJ, Papotti M, Sasano H, Volante M. Immunohistochemical Biomarkers of Adrenal Cortical Neoplasms. Endocr Pathol. 2018;29:137-149.
Kimura N, Shiga K, Kaneko K, Sugisawa C, Katabami T, Naruse M. The Diagnostic Dilemma of GATA3 Immunohistochemistry in Pheochromocytoma and Paraganglioma. Endocr Pathol 2020;31:95-100.
Miettinen M, McCue PA, Sarlomo-Rikala M, Rys J, Czapiewski P, Wazny K, Renata Langfort, Piotr Waloszczyk, Wojciech Biernat, Jerzy Lasota, Zengfeng Wang. GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelial tumors. Am J Surg Pathol 2014;38:13–22.
Mete O, Kefeli M, Çalışkan S, Asa SL. GATA3 immunoreactivity expands the transcription factor profile of pituitary neuroendocrine tumors. Mod Pathol. 2019;32:484-489.
Turchini J, Sioson L, Clarkson A, Sheen A, Gill AJ. Utility of GATA-3 Expression in the Analysis of Pituitary Neuroendocrine Tumour (PitNET) Transcription Factors. Endocr Pathol. 2020;31:150-155.
Erickson LA, Mete O. Immunohistochemistry in Diagnostic Parathyroid Pathology. Endocr Pathol. 2018;29(2):113-129.
Asa SL, Arkun K, Tischler AS, Qamar A, Deng FM, Perez-Ordonez B, Weinreb I, Bishop JA, Wenig BM, Mete O. Middle Ear “Adenoma”: a Neuroendocrine Tumor with Predominant L Cell Differentiation. Endocr Pathol. 2021;32(4):433-441.
Kimura N, Miura Y, Nagatsu I, Nagura H. Catecholamine synthesizing enzymes in 70 cases of functioning and non-functioning phaeochromocytoma and extra-adrenal paraganglioma. Virchows Arch A Pathol Anat Histopathol 1992;421:25-32.
Kimura N, Shiga K, Kaneko KI, Oki Y, Sugisawa C, Saito J, Tawara S, Akahori H, Sogabe S, Yamashita T, Takekoshi K, Naruse M, Katabami T. Immunohistochemical Expression of Choline Acetyltransferase and Catecholamine-Synthesizing Enzymes in Head-and-Neck and Thoracoabdominal Paragangliomas and Pheochromocytomas. Endocr Pathol. 2021;32:442-451.
Osinga TE, Korpershoek E, de Krijger RR, Kerstens MN, Dullaart RP, Kema IP, van der Laan BF, van der Horst-Schrivers AN, Links TP. Catecholamine-Synthesizing Enzymes Are Expressed in Parasympathetic Head and Neck Paraganglioma Tissue. Neuroendocrinology. 2015;101:289-295.
Kimura N. Dopamine beta-hydroxylase: An Essential and Optimal Immunohistochemical Marker for Pheochromocytoma and Sympathetic Paraganglioma. Endocr Pathol. 2021;32:258-261.
Timmers, HJLM, Pacak, K, Huynh, TT, Abu-Asab, M, Tsokos, M, Merino, MJ, Baysal, BE, Adams, KT, Eisenhofer, G. Biochemically silent abdominal paragangliomas in patients with mutations in the succinate dehydrogenase subunit B gene. J Clin Endocrinol Metab 2008;93:4826–4832.
Matsuda, Y, Kimura, N, Yoshimoto, T, Sekiguchi, Y, Tomoishi, J, Kasahara, I, Hara, Y, Ogawa, Y. Dopamine-secreting paraganglioma in the retroperitoneum. Endocr Pathol. 2017;28:36–40.
Miyamoto S, Yoshida Y, Ozeki Y, Okamoto M, Gotoh K, Masaki T, Nishida H, Shibuya T, Shin T, Daa T, Mimata H, Kimura N, Shibata H. Dopamine-Secreting Pheochromocytoma and Paraganglioma. J Endocr Soc 2021;5:bvab163.
Powers JF, Tischler AS. Immunohistochemical Staining for SOX10 and SDHB in SDH-Deficient Paragangliomas Indicates that Sustentacular Cells Are Not Neoplastic. Endocr Pathol. 2020; 31:307-309.
Zhou YY, Coffey M, Mansur D, Wasman J, Asa SL, Couce M. Images in Endocrine Pathology: Progressive Loss of Sustentacular Cells in a Case of Recurrent Jugulotympanic Paraganglioma over a Span of 5 years. Endocr Pathol. 2020;31:310-314.
Thai E, Gnetti L, Gilli A, Caruana P, Dalla Valle R, Buti S. Very late recurrence of an apparently benign pheochromocytoma. J Cancer Res Ther. 2015;11:1036.
Thompson LD. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551-66.
Gao B, Meng F, Bian W, Chen J, Zhao H, Ma G, Shi B, Zhang J, Liu Y, Xu Z. Development and validation of pheochromocytoma of the adrenal gland scaled score for predicting malignant pheochromocytomas. Urology. 2006;68:282-286.
Wu D, Tischler AS, Lloyd RV, DeLellis RA, de Krijger R, van Nederveen F, Nosé V. Observer variation in the application of the Pheochromocytoma of the Adrenal Gland Scaled Score. Am J Surg Pathol. 2009;33:599-608.
Wachtel H, Hutchens T, Baraban E, Schwartz LE, Montone K, Baloch Z, LiVolsi V, Krumeich L, Fraker DL, Nathanson KL, Cohen DL, Fishbein L. Predicting Metastatic Potential in Pheochromocytoma and Paraganglioma: A Comparison of PASS and GAPP Scoring Systems. J Clin Endocrinol Metab. 2020;105:e4661–70.
Stenman A, Zedenius J, Juhlin CC. The Value of Histological Algorithms to Predict the Malignancy Potential of Pheochromocytomas and Abdominal Paragangliomas-A Meta-Analysis and Systematic Review of the Literature. Cancers (Basel). 2019;11(2):225.eat
Kimura N, Takayanagi R, Takizawa N, Itagaki E, Katabami T, Kakoi N, Rakugi H, Ikeda Y, Tanabe A, Nigawara T, Ito S, Kimura I, Naruse M; Phaeochromocytoma Study Group in Japan. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:405–14.
Koh JM, Ahn SH, Kim H, Kim BJ, Sung TY, Kim YH, Hong SJ, Song DE, Lee SH. Validation of pathological grading systems for predicting metastatic potential in pheochromocytoma and paraganglioma. PLoS One. 2017;12:e0187398.
Eisenhofer G, Tischler AS. Neuroendocrine cancer. Closing the GAPP on predicting metastases. Nat Rev Endocrinol. 2014;10:315–316.
Pierre C, Agopiantz M, Brunaud L, Battaglia-Hsu SF, Max A, Pouget C, Nomine C, Lomazzi S, Vignaud JM, Weryha G, Oussalah A, Gauchotte G, Busby-Venner H. COPPS, a composite score integrating pathological features, PS100 and SDHB losses, predicts the risk of metastasis and progression-free survival in pheochromocytomas/paragangliomas. Virchows Arch. 2019;474:721-734.
Tischler AS, deKrijger RR. 15 YEARS OF PARAGANGLIOMA: Pathology of pheochromocytoma and paraganglioma. Endocr Relat Cancer. 2015;22:T123-33.
Stenman A, Zedenius J, Juhlin CC. Over-diagnosis of potential malignant behavior in MEN 2A-associated pheochromocytomas using the PASS and GAPP algorithms. Langenbecks Arch Surg. 2018;403:785-790.
Juhlin CC. Challenges in Paragangliomas and Pheochromocytomas: from Histology to Molecular Immunohistochemistry. Endocr Pathol. 2021;32:228-244.
Thompson LDR, Gill AJ, Asa SL, Clifton-Bligh RJ, de Krijger RR, Kimura N, Komminoth P, Lack EE, Lenders JWM, Lloyd RV, Papathomas TG, Sadow PM, Tischler AS. Data set for the reporting of pheochromocytoma and paraganglioma: explanations and recommendations of the guidelines from the International Collaboration on Cancer Reporting. Hum Pathol. 2021;110:83-97.
Jochmanova I, Abcede AMT, Guerrero RJS, Malong CLP, Wesley R, Huynh T, Gonzales MK, Wolf KI, Jha A, Knue M, Prodanov T, Nilubol N, Mercado-Asis LB, Stratakis CA, Pacak K. Clinical characteristics and outcomes of SDHB-related pheochromocytoma and paraganglioma in children and adolescents. J Cancer Res Clin Oncol. 2020;146:1051-1063.
Job S, Draskovic I, Burnichon N, Buffet A, Cros J, Lépine C, Venisse A, Robidel E, Verkarre V, Meatchi T, Sibony M, Amar L, Bertherat J, de Reyniès A, Londoño-Vallejo A, Favier J, Castro-Vega LJ, Gimenez-Roqueplo AP. Telomerase Activation and ATRX Mutations Are Independent Risk Factors for Metastatic Pheochromocytoma and Paraganglioma. Clin Cancer Res. 2019;25:760-770.
Fishbein L, Leshchiner I, Walter V, Danilova L, Robertson AG, Johnson AR, Lichtenberg TM, Murray BA, Ghayee HK, Else T, Ling S, Jefferys SR, de Cubas AA, Wenz B, Korpershoek E, Amelio AL, Makowski L, Rathmell WK, Gimenez-Roqueplo AP, Giordano TJ, Asa SL, Tischler AS; Cancer Genome Atlas Research Network, Pacak K, Nathanson KL, Wilkerson MD. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer Cell. 2017;31:181–193.
Millar AC, Mete O, Cusimano RJ, Fremes SE, Keshavjee S, Morgan CD, Asa SL, Ezzat S, Gilbert J. Functional cardiac paraganglioma associated with a rare SDHC mutation. Endocr Pathol. 2014;25:315-320.
Unger P, Hoffman K, Pertsemlidis D, Thung S, Wolfe D, Kaneko M. S100 protein-positive sustentacular cells in malignant and locally aggressive adrenal pheochromocytomas. Arch Pathol Lab Med. 1991;115:484-487.
Jimenez C, Libutti SK, Landry CS et al. Adrenal-neuroendocrine tumors. In: Amin MB, Edge S, Greene F, et al. eds. AJCC Cancer Staging Manual. 8 ed. New York: Springer, 2017; 919-927.
Ayala-Ramirez M, Palmer JL, Hofmann MC, de la Cruz M, Moon BS, Waguespack SG, Habra MA, Jimenez C. Bone metastases and skeletal-related events in patients with malignant pheochromocytoma and sympathetic paraganglioma. J Clin Endocrinol Metab. 2013;98:1492-1497.
Ayala-Ramirez M, Feng L, Johnson MM, Ejaz S, Habra MA, Rich T, Busaidy N, Cote GJ, Perrier N, Phan A, Patel S, Waguespack S, Jimenez C. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. 2011;96:717-725.
Eisenhofer G, Lenders JW, Siegert G, Bornstein SR, Friberg P, Milosevic D, Mannelli M, Linehan WM, Adams K, Timmers HJ, Pacak K. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 2012;48:1739-1749.
Amar L, Baudin E, Burnichon N, Peyrard S, Silvera S, Bertherat J, Bertagna X, Schlumberger M, Jeunemaitre X, Gimenez-Roqueplo AP, Plouin PF. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007;92:3822–3828.'
Cui Y, Ma X, Gao Y, Chang X, Chen S, Lu L, Tong A. Local-Regional Recurrence of Pheochromocytoma/Paraganglioma: Characteristics, Risk Factors and Outcomes. Front Endocrinol (Lausanne). 2021;12:762548.
Mizuno K, Ueno Y. Autonomic Nervous System and the Liver. Hepatol Res. 2017;47:160-165.
Ellis RJ, Patel D, Prodanov T, Nilubol N, Pacak K, Kebebew E. The presence of SDHB mutations should modify surgical indications for carotid body paragangliomas. Ann Surg. 2014;260:158-162.
Abdel-Rahman O. Assessment of the AJCC staging system of pheochromocytomas/paragangliomas. Endocrine. 2021 Aug 27. doi: https://doi.org/10.1007/s12020-021-02854-3. Epub ahead of print.
Papathomas TG, Suurd DPD, Pacak K, Tischler AS, Vriens MR, Lam AK, de Krijger RR. What Have We Learned from Molecular Biology of Paragangliomas and Pheochromocytomas? Endocr Pathol. 2021;32(1):134-153.
Pamporaki C, Hamplova B, Peitzsch M, Prejbisz A, Beuschlein F, Timmers HJLM, Fassnacht M, Klink B, Lodish M, Stratakis CA, Huebner A, Fliedner S, Robledo M, Sinnott RO, Januszewicz A, Pacak K, Eisenhofer G. Characteristics of Pediatric vs Adult Pheochromocytomas and Paragangliomas. J Clin Endocrinol Metab. 2017;102:1122-1132.
Mete O, Tischler AS, Asa SL. Paragangliomas and pheochromocytomas. In: Asa SL, La Rosa S, Mete O (eds). The spectrum of neuroendocrine neoplasia. Springer, Cham, pp: 263–285.
van Nederveen FH, Korpershoek E, Lenders JW, de Krijger RR, Dinjens WN. Somatic SDHB mutation in an extraadrenal pheochromocytoma. N Engl J Med. 2007;357:306-308.
Duan K, Mete O. Familial endocrine tumor syndromes: Clinical and predictive roles of molecular histopathology. AJSP: Reviews and Reports. 2017; 22:246–268.
Eisenhofer G, Tischler AS, de Krijger RR. Diagnostic tests and biomarkers for pheochromocytoma and extra-adrenal paraganglioma: from routine laboratory methods to disease stratification. Endocr Pathol. 2012;23:4-14.
Oudijk L, Gaal J, de Krijger RR. The Role of Immunohistochemistry and Molecular Analysis of Succinate Dehydrogenase in the Diagnosis of Endocrine and Non-Endocrine Tumors and Related Syndromes. Endocr Pathol. 2019;30:64-73.
Korpershoek E, Favier J, Gaal J, Burnichon N, van Gessel B, Oudijk L, Badoual C, Gadessaud N, Venisse A, Bayley JP, van Dooren MF, de Herder WW, Tissier F, Plouin PF, van Nederveen FH, Dinjens WN, Gimenez-Roqueplo AP, de Krijger RR. SDHA immunohistochemistry detects germline SDHA gene mutations in apparently sporadic paragangliomas and pheochromocytomas. J Clin Endocrinol Metab. 2011;96:E1472-6.
Papathomas TG, Oudijk L, Persu A, Gill AJ, van Nederveen F, Tischler AS, Tissier F, Volante M, Matias-Guiu X, Smid M, Favier J, Rapizzi E, Libe R, Currás-Freixes M, Aydin S, Huynh T, Lichtenauer U, van Berkel A, Canu L, Domingues R, Clifton-Bligh RJ, Bialas M, Vikkula M, Baretton G, Papotti M, Nesi G, Badoual C, Pacak K, Eisenhofer G, Timmers HJ, Beuschlein F, Bertherat J, Mannelli M, Robledo M, Gimenez-Roqueplo AP, Dinjens WN, Korpershoek E, de Krijger RR. SDHB/SDHA immunohistochemistry in pheochromocytomas and paragangliomas: a multicenter interobserver variation analysis using virtual microscopy: a Multinational Study of the European Network for the Study of Adrenal Tumors (ENS@T). Mod Pathol. 2015;28:807-821.
Dwight T, Mann K, Benn DE, Robinson BG, McKelvie P, Gill AJ, Winship I, Clifton-Bligh RJ. Familial SDHA mutation associated with pituitary adenoma and pheochromocytoma/paraganglioma. J Clin Endocrinol Metab. 2013;98:E1103-8.
Turchini J, Gill AJ. Morphologic Clues to Succinate Dehydrogenase (SDH) Deficiency in Pheochromocytomas and Paragangliomas. Am J Surg Pathol. 2020;44:422-424.
Mete O, Hannah-Shmouni F, Kim R, Stratakis CA. Inherited neuroendocrine neoplasms. In: Asa SL, La Rosa S, Mete O (eds). The spectrum of neuroendocrine neoplasia. Springer, Cham, pp: 409–459.
Gill AJ. Succinate dehydrogenase (SDH)-deficient neoplasia. Histopathology. 2018;72:106-116.
van Nederveen FH, Gaal J, Favier J, Korpershoek E, Oldenburg RA, de Bruyn EM, Sleddens HF, Derkx P, Rivière J, Dannenberg H, Petri BJ, Komminoth P, Pacak K, Hop WC, Pollard PJ, Mannelli M, Bayley JP, Perren A, Niemann S, Verhofstad AA, de Bruïne AP, Maher ER, Tissier F, Méatchi T, Badoual C, Bertherat J, Amar L, Alataki D, Van Marck E, Ferrau F, François J, de Herder WW, Peeters MP, van Linge A, Lenders JW, Gimenez-Roqueplo AP, de Krijger RR, Dinjens WN. An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: a retrospective and prospective analysis. Lancet Oncol. 2009;10:764-771.
Castro-Vega LJ, Buffet A, De Cubas AA, Cascón A, Menara M, Khalifa E, Amar L, Azriel S, Bourdeau I, Chabre O, Currás-Freixes M, Franco-Vidal V, Guillaud-Bataille M, Simian C, Morin A, Letón R, Gómez-Graña A, Pollard PJ, Rustin P, Robledo M, Favier J, Gimenez-Roqueplo AP. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum Mol Genet. 2014;23:2440-2446.
Udager AM, Magers MJ, Goerke DM, Vinco ML, Siddiqui J, Cao X, Lucas DR, Myers JL, Chinnaiyan AM, McHugh JB, Giordano TJ, Else T, Mehra R. The utility of SDHB and FH immunohistochemistry in patients evaluated for hereditary paraganglioma-pheochromocytoma syndromes. Hum Pathol. 2018;71:47-54.
Favier J, Meatchi T, Robidel E, Badoual C, Sibony M, Nguyen AT, Gimenez-Roqueplo AP, Burnichon N. Carbonic anhydrase 9 immunohistochemistry as a tool to predict or validate germline and somatic VHL mutations in pheochromocytoma and paraganglioma-a retrospective and prospective study. Mod Pathol. 2020;33:57-64.
Cassol C, Mete O. Endocrine manifestations of von Hippel-Lindau disease. Arch Pathol Lab Med. 2015;139:263-268.
Eisenhofer G, Gimenez-Roqueplo AP, Robledo M. MAX mutations cause hereditary and sporadic pheochromocytoma and paraganglioma. Clin Cancer Res. 2012;18:2828-37.
Korpershoek E, Koffy D, Eussen BH, Oudijk L, Papathomas TG, van Nederveen FH, Belt EJ, Franssen GJ, Restuccia DF, Krol NM, van der Luijt RB, Feelders RA, Oldenburg RA, van Ijcken WF, de Klein A, de Herder WW, de Krijger RR, Dinjens WN. Complex MAX Rearrangement in a Family With Malignant Pheochromocytoma, Renal Oncocytoma, and Erythrocytosis. J Clin Endocrinol Metab. 2016;101:453-460.
Seabrook AJ, Harris JE, Velosa SB, Kim E, McInerney-Leo AM, Dwight T, Hockings JI, Hockings NG, Kirk J, Leo PJ, Love AJ, Luxford C, Marshall M, Mete O, Pennisi DJ, Brown MA, Gill AJ, Hockings GI, Clifton-Bligh RJ, Duncan EL. Multiple Endocrine Tumors Associated with Germline MAX Mutations: Multiple Endocrine Neoplasia Type 5? J Clin Endocrinol Metab. 2021;106:1163-1182.
Cheung VKY, Gill AJ, Chou A. Old, New, and Emerging Immunohistochemical Markers in Pheochromocytoma and Paraganglioma. Endocr Pathol. 2018;29:169-175.
Maffeis V, Cappellesso R, Nicolè L, Guzzardo V, Menin C, Elefanti L, Schiavi F, Guido M, Fassina A. Loss of BAP1 in Pheochromocytomas and Paragangliomas Seems Unrelated to Genetic Mutations. Endocr Pathol. 2019;30:276-284.
Gupta S, Zhang J, Erickson LA. Composite Pheochromocytoma/Paraganglioma-Ganglioneuroma: A Clinicopathologic Study of Eight Cases with Analysis of Succinate Dehydrogenase. Endocr Pathol. 2017;28:269-275.
Kikuchi Y, Wada R, Sakihara S, Suda T, Yagihashi S. Pheochromocytoma with histologic transformation to composite type, complicated by watery diarrhea, hypokalemia, and achlorhydria syndrome. Endocr Pract. 2012;18:e91-6.
Ende K, Henkel B, Brodhun M, Salomon C, Lauten P, Conrad E, Seifert M, Stier A, Scharf JG. A 45-year-old female with hypokalemic rhabdomyolysis due to VIP-producing composite pheochromocytoma. Z Gastroenterol. 2012;50:589-594.
George DJ, Watermeyer GA, Levin D, Epstein D, Ross IL, Scholz BU, Setshedi M, Locketz M, Dittrich C, Shaw J, Krige JE. Composite adrenal phaeochromocytoma-ganglioneuroma causing watery diarrhoea, hypokalaemia and achlorhydria syndrome. Eur J Gastroenterol Hepatol. 2010;22:632-4.
Tran L, Fitzpatrick C, Cohn SL, Pytel P. Composite tumor with pheochromocytoma and immature neuroblastoma: report of two cases with cytogenetic analysis and discussion of current terminology. Virchows Arch. 2017;471:553-557.
Satake H, Inoue K, Kamada M, Watanabe H, Furihata M, Shuin T. Malignant composite pheochromocytoma of the adrenal gland in a patient with von Recklinghausen's disease. J Urol. 2001;165:1199-200.
Ch'ng ES, Hoshida Y, Iizuka N, Morii E, Ikeda JI, Yamamoto A, Tomita Y, Hanasaki H, Katsuya T, Maeda K, Ohishi M, Rakugi H, Ogihara T, Aozasa K. Composite malignant pheochromocytoma with malignant peripheral nerve sheath tumour: a case with 28 years of tumour-bearing history. Histopathology. 2007;51(3):420-422.
Franquemont DW, Mills SE, Lack EE. Immunohistochemical detection of neuroblastomatous foci in composite adrenal pheochromocytoma-neuroblastoma. Am J Clin Pathol. 1994;102:163-170.
Rizzo S, Bonomo S, Moser A, Bottura D, Castellini C, Mazzola F, Lauro E, Vicenzi L, Betresini B, Angeli G, Brazzarola P, D'Azzò G, Rosa G. Feocromocitoma bilaterale associato a GIST duodeno-digiunale in paziente affetto da malattia di von Recklinghausen: presentazione di un caso clinico [Bilateral pheochromocytoma associated with duodeno-jejunal GIST in patient with von Recklinghausen disease: report of a clinical case]. Chir Ital. 2001;53(2):243-246.
Pozza C, Sesti F, Di Dato C, Sbardella E, Pofi R, Schiavi F, Bonifacio V, Isidori AM, Faggiano A, Lenzi A, Giannetta E. A Novel MAX Gene Mutation Variant in a Patient With Multiple and “Composite” Neuroendocrine-Neuroblastic Tumors. Front Endocrinol (Lausanne). 2020;11:234.
Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B. Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee. Cancer. 1999;86(2):349-363.
Miettinen M, Chatten J, Paetau A, Stevenson A. Monoclonal antibody NB84 in the differential diagnosis of neuroblastoma and other small round cell tumors. Am J Surg Pathol. 1998;22:327-332.
Bielle F, Fréneaux P, Jeanne-Pasquier C, Maran-Gonzalez A, Rousseau A, Lamant L, Paris R, Pierron G, Nicolas AV, Sastre-Garau X, Delattre O, Bourdeaut F, Peuchmaur M. PHOX2B immunolabeling: a novel tool for the diagnosis of undifferentiated neuroblastomas among childhood small round blue-cell tumors. Am J Surg Pathol. 2012;36:1141-1149.
Hata JL, Correa H, Krishnan C, Esbenshade AJ, Black JO, Chung DH, Mobley BC. Diagnostic utility of PHOX2B in primary and treated neuroblastoma and in neuroblastoma metastatic to the bone marrow. Arch Pathol Lab Med. 2015;139:543-546.
Chan WH, Anderson CR, Gonsalvez DG. From proliferation to target innervation: signaling molecules that direct sympathetic nervous system development. Cell Tissue Res. 2018;372:171-193.
Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224:1121-1124.
Seeger RC, Brodeur GM, Sather H, Dalton A, Siegel SE, Wong KY, Hammond D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313:1111-1116.
Wang LL, Suganuma R, Ikegaki N, Tang X, Naranjo A, McGrady P, London WB, Hogarty MD, Gastier-Foster JM, Look AT, Park JR, Maris JM, Cohn SL, Seeger RC, Shimada H. Neuroblastoma of undifferentiated subtype, prognostic significance of prominent nucleolar formation, and MYC/MYCN protein expression: a report from the Children’s Oncology Group. Cancer. 2013;119:3718-3726.
Carpenter EL, Mossé YP. Targeting ALK in neuroblastoma--preclinical and clinical advancements. Nat Rev Clin Oncol. 2012;9:391-399.
Kamihara J, Bourdeaut F, Foulkes WD, Molenaar JJ, Mossé YP, Nakagawara A, Parareda A, Scollon SR, Schneider KW, Skalet AH, States LJ, Walsh MF, Diller LR, Brodeur GM. Retinoblastoma and Neuroblastoma Predisposition and Surveillance. Clin Cancer Res. 2017;23:e98-e106.
Mossé YP. Anaplastic Lymphoma Kinase as a Cancer Target in Pediatric Malignancies. Clin Cancer Res. 2016;22:546-552.
Javanmardi N, Fransson S, Djos A, Sjöberg RM, Nilsson S, Truvé K, Kogner P, Martinsson T. Low Frequency ALK Hotspots Mutations In Neuroblastoma Tumours Detected By Ultra-deep Sequencing: Implications For ALK Inhibitor Treatment. Sci Rep. 2019;9:2199.
Schulte JH, Schulte S, Heukamp LC, Astrahantseff K, Stephan H, Fischer M, Schramm A, Eggert A. Targeted Therapy for Neuroblastoma: ALK Inhibitors. Klin Padiatr. 2013;225:303-308.
Schleiermacher G, Mosseri V, London WB, Maris JM, Brodeur GM, Attiyeh E, Haber M, Khan J, Nakagawara A, Speleman F, Noguera R, Tonini GP, Fischer M, Ambros I, Monclair T, Matthay KK, Ambros P, Cohn SL, Pearson AD. Segmental chromosomal alterations have prognostic impact in neuroblastoma: a report from the INRG project. Br J Cancer. 2012;107:1418-1422.
Koneru B, Lopez G, Farooqi A, Conkrite KL, Nguyen TH, Macha SJ, Modi A, Rokita JL, Urias E, Hindle A, Davidson H, Mccoy K, Nance J, Yazdani V, Irwin MS, Yang S, Wheeler DA, Maris JM, Diskin SJ, Reynolds CP. Telomere Maintenance Mechanisms Define Clinical Outcome in High-Risk Neuroblastoma. Cancer Res. 2020;80:2663-2675.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent for Publication
All authors consent to publication.
Competing Interests
Dr. Ozgur Mete is the Editor-in-Chief of Endocrine Pathology. This review article was handled by an independent senior editor and peer-reviewed as per the journal standards. The remaining authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ronald R. de Krijger and Arthur Tischler are co-senior authors in this manuscript.
Rights and permissions
About this article
Cite this article
Mete, O., Asa, S.L., Gill, A.J. et al. Overview of the 2022 WHO Classification of Paragangliomas and Pheochromocytomas. Endocr Pathol 33, 90–114 (2022). https://doi.org/10.1007/s12022-022-09704-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-022-09704-6