Abstract
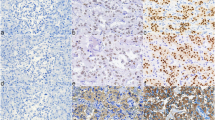
Paragangliomas (PGLs) are neural-crest-derived, non-epithelial neuroendocrine tumors distributed along the parasympathetic and sympathetic nerves. Head-and-neck PGLs (HNPGLs) have been recognized as nonchromaffin, nonfunctional, parasympathetic tumors. By contrast, thoracoabdominal paragangliomas and pheochromocytomas (PPGLs) are chromaffin, functional, sympathetic tumors. Although HNPGLs and PPGLs have the same histological structure, the zellballen pattern, composed of chief and sustentacular cells surrounded by abundant capillaries, the pathobiological differences between these types of PGLs remain unclarified. To determine the phenotypic features of these PGLs, we performed an immunohistochemical study using specific antibodies against choline acetyltransferase (ChAT), an enzyme involved in acetylcholine synthesis, and enzymes for the catecholamine-synthesis, tyrosine hydroxylase (TH), and dopamine beta-hydroxylase (DBH), in 34 HNPGLs from 31 patients, 12 thoracoabdominal PGLs from 12 patients, and 26 pheochromocytomas from 22 patients. The expression of ChAT, TH, and DBH was 100%, 23%, and 10% in the HNPGLs; 12%, 100%, and 100% in the pheochromocytomas; and 25%, 67%, and 100% in the thoracoabdominal PGLs, respectively. These results designate HNPGLs as acetylcholine-producing parasympathetic tumors, in contrast to PPGLs being catecholamine-producing tumors. The other most frequently used neuroendocrine markers are synaptophysin and chromogranin A expressed 100% and 80%, respectively, and synaptophysin was superior to chromogranin A in HNPGLs. This is the first report of HNPGLs being acetylcholine-producing tumors. Immunohistochemistry of ChAT could be greatly useful for pathologic diagnosis of HNPGL. Whether measurement of acetylcholine levels in the blood or urine could be a tumor marker of HNPGLs should be investigated soon.



Similar content being viewed by others
References
Valero C, Ganly I, Shah JP (2020)Head and neck paragangliomas: 30-year experience. Head Neck 42: 2486–2495. https://doi.org/10.1002/hed.26277
Garcia-Carbonero R, Matute Teresa F, Mercader-Cidoncha E, Mitjavila-Casanovas M, Robledo M, Tena I, Alvarez-Escola C, Arístegui M, Bella-Cueto MR, Ferrer-Albiach C, Hanzu FA (2021) Multidisciplinary practice guidelines for the diagnosis, genetic counseling and treatment of pheochromocytomas and paragangliomas. Clin Transl Oncol 23:1995–2019. https://doi.org/10.1007/s12094-021-02622-9
Williams MD (2017) Paragangliomas of the Head and Neck: An Overview from Diagnosis to Genetics. Head Neck Pathol 3:278–287. https://doi.org/10.1007/s12105-017-0803-4
Kimura N, Capella C, Gill A, Komminoth P, Lam AKY,Tischler AS, Williams MD (2017) Paraganglion tumours. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ (ed) WHO Classi-fication of Head and Neck Tumours. 4th eds. IARC Press, Lyon, pp 276–284
Kimura N, Capella C, De Lellis R, Epstein J, Gill A, Kawashima A, Koch C, Komminoth P, Lam A, Merino M et al (2017) Extraadrenal paragangliomas. In Lloyd RV, Osamura RY, Kloppel G, Rosai J. (ed) WHO Classifications of Tumours of Endocrine Organs. 4th edn. IARC Press, Lyon, pp190-195
Kimura H, McGeer PL, Peng F, McGeer EG (1980) Choline acetyltransferase-containing neurons in rodent brain demonstrated by immunohistochemistry. Science 208:1057–1059. https://doi.org/10.1126/science
Eckenstein F, Barde YA, Thoenen H (1981) Production of specific antibodies to choline acetyltransferase purified from pig brain. Neuroscience 6:993–1000. https://doi.org/10.1016/0306-4522(81)90065-8
Levey, A. I., Aoki, M., Fitch, F. W., and Wainer, B. H (1981) The production of monoclonal antibodies reactive with bovine choline acetyltransferase. Brain Res 218:383–387. https://doi.org/10.1016/0006-8993(81)91316-0
Porter AJ, Wattchow DA, Brookes SJ, Schemann M, Costa M (1996) Choline acetyltransferase immunoreactivity in the human small and large intestine. Gastroenterology 111:401–408. https://doi.org/10.1053/gast.1996.v111
Bellier JP, Kimura H (2011) Peripheral type of choline acetyltransferase: biological and evolutionary implications for novel mechanisms in cholinergic system. J Chem Neuroanat 42:225–235. https://doi.org/10.1016/j.jchemneu.2011.02.005
Bellier JP, Yuan PQ, Mukaisho K, Tooyama I, Taché Y, Kimura H (2019) A Novel antiserum against a predicted human peripheral choline acetyltransferase (hpChAT) for labeling neuronal structures in human colon. Front Neuroanat 13:37. https://doi.org/10.3389/fnana.2019.00037
Kimura N, Sasano N, Miura Y, Kobayashi K (1984) Adrenal and extra-adrenal pheochromocytomas: an ultrastructural and formaldehyde-induced fluorescence study with catecholamine content. Tohoku J Exp Med 142:1-14. https://doi.org/10.1620/tjem.142.1
Matsumoto S, Tanaka K, Yamamoto A, Nakada H, Uchida M, Tashiro Y (1987) Immunoelectron microscopic localization of dopamine beta-hydroxylase and chromogranin A in adrenomedullary chromaffin cells. Cell Struct Funct. 12:483-96. https://doi.org/10.1247/csf.12.483
Wiedenmann B, Franke WW, Kuhn C, Moll R, Gould VE (1986) Synaptophysin: a marker protein for neuroendocrine cells and neoplasms. Proc Natl Acad Sci USA 83:3500-4. https://doi.org/10.1073/pnas.83.10.3500
Wiedenmann B, Huttner WB (1989) Synaptophysin and chromogranins/secretogranins--widespread constituents of distinct types of neuroendocrine vesicles and new tools in tumor diagnosis. Virchows Arch B Cell Pathol Incl Mol Pathol 58:95-121. https://doi.org/10.1007/BF02890062
Kimura N, Sasano N, Yamada R, Satoh J (1988) Immunohistochemical study of chromogranin in 100 cases of pheochromocytoma, carotid body tumour, medullary thyroid carcinoma and carcinoid tumour. Virchows Arch A Pathol Anat Histopathol 413:33-38. https://doi.org/10.1007/BF00844279
Kimura N, Miura W, Noshiro T, Mizunashi K, Hanew K, Shimizu K, Watanabe T, Shibukawa S, Sohn HE, Abe K, Miura Y, Nagura H (1997) Plasma chromogranin A in pheochromocytoma, primary hyperparathyroidism and pituitary adenoma in com-parison with catecholamine, parathyroid hormone and pituitary hormones. Endocr J 44:319–27. https://doi.org/10.1507/endocrj.44.319
Bílek R, Vlček P, Šafařík L, Michalský D, Novák K, Dušková J, Václavíková E, Widimský J Jr, Zelinka T (2019) Chromogranin A in the Laboratory Diagnosis of Pheochromocytoma and Paraganglioma. Cancers (Basel). 11:586. https://doi.org/10.3390/cancers11040586
Kimura N, Takayanagi R, Takizawa N, Itagaki E, Katabami T, Kakoi N, Rakugi H, Ikeda Y, Tanabe A, Nigawara T, Ito S, Kimura I, Naruse M (2014) Phaeochromocytoma Study Group in Japan. Pathological grading for predicting metastasis in phaeochromo-cytoma and paraganglioma. Endocr Relat Cancer 21:405-14. https://doi.org/10.1530/ERC-13-0494
Kimura N (2021) Dopamine beta-hydroxylase: An essential and optimal immunohistochemical marker for pheochromocytoma and sympathetic paraganglioma. Endocr Pathol 32:258-261. https://doi.org/10.1007/s12022-020-09655-w
Buffet A, Burnichon N, Favier J, Gimenez-Roqueplo AP (2020). An overview of 20 years of genetic studies in pheochromocytoma and paraganglioma. Best Pract Res Clin Endocrinol Metab 34: 101416. https://doi.org/10.1016/j.beem.2020.101416
Kimura N, Takekoshi K, Horii A, Morimoto R, Imai T, Oki Y, Saito T, Midorikawa S, Arao T, Sugisawa C, Yamada M, Otuka Y, Kurihara I, Sugano K, Nakane M, Fukuuchi A, Kitamoto T, Saito J, Nishikawa T, Naruse M (2014) Clinicopathological study of SDHB mutation-related pheochromocytoma and sympathetic paraganglioma. Endocr Relat Cancer 21: L13–6. https://doi.org/10.1530/ERC-13-0530
van Nederveen FH, Gaal J, Favier J, Korpershoek E, Oldenburg RA, de Bruyn EM, Sleddens HF, Derkx P, Rivière J, Dannenberg H, Petri BJ, Komminoth P, Pacak K, Hop WC, Pollard PJ, Mannelli M, Bayley JP, Perren A, Niemann S, Verhofstad AA, de Bruïne AP, Maher ER, Tissier F, Méatchi T, Badoual C, Bertherat J, Amar L, Alataki D, Van Marck E, Ferrau F, François J, de Herder WW, Peeters MP, van Linge A, Lenders JW, Gimenez-Roqueplo AP, de Krijger RR, Dinjens WN (2009) Lancet Oncol 10: 764-71. https://doi.org/10.1016/S1470-2045(09)70164-0
Papathomas TG, Oudijk L, Persu A, Gill AJ, van Nederveen F, Tischler AS, Tissier F, Volante M, Matias-Guiu X, Smid M, Favier J, Rapizzi E, Libe R, Currás-Freixes M, Aydin S, Huynh T, Lichtenauer U, van Berkel A, Canu L, Domingues R, Clifton-Bligh RJ, Bialas M, Vikkula M, Baretton G, Papotti M, Nesi G, Badoual C, Pacak K, Eisenhofer G, Timmers HJ, Beuschlein F, Bertherat J, Mannelli M, Robledo M, Gimenez-Roqueplo AP, Dinjens WN, Korpershoek E, de Krijger RR (2015) SDHB/SDHA immunohistochemistry in pheochromocytomas and paragangliomas: a multicenter interobserver variation analysis using virtual microscopy: a Multinational Study of the European Network for the Study of Adrenal Tumors (ENS@T). Mod Pathol 28:807-21. https://doi.org/10.1038/modpathol.2015.41
Favier J, Meatchi T, Robidel E, Badoual C, Sibony M, Nguyen AT, Gimenez-Roqueplo AP, Burnichon N (2020) Carbonic anhydrase 9 immunohistochemistry as a tool to predict or validate germline and somatic VHL mutations in pheochromocytoma and paraganglioma-a retrospective and prospective study. Mod Pathol 33: 57–64. https://doi.org/10.1038/s41379-019-0343-4
Astrom K, Cohen JE, Willett-Brozick JE, Aston CE, Baysal BE (2003) Altitude is a phenotypic modifier in hereditary paraganglioma type 1: evidence for an oxygen-sensing defect. Human Genetics.113: 228–37. https://doi.org/10.1007/s00439-003-0969-6
Opotowsky AR, Moko LE, Ginns J, Rosenbaum M, Greutmann M, Aboulhosn J, Hageman A, Kim Y, Deng LX, Grewal J, Zaidi AN, Almansoori G, Oechslin E, Earing M, Landzberg MJ, Singh MN, Wu F, Vaidya A (2015) Pheochromocytoma and paraganglioma in cyanotic congenital heart disease. J Clin Endocrinol Metab 100:1325-34. https://doi.org/10.1210/jc.2014-3863
Osinga TE, Korpershoek E, de Krijger RR, Kerstens MN, Dullaart RP, Kema IP, van der Laan BF, van der Horst-Schrivers AN, Links TP (2015) Catecholamine-synthesizing enzymes are expressed in parasympathetic head and neck paraganglioma tissue. Neuroendocrinology. 101: 289-95. https://doi.org/10.1159/000377703
Acknowledgements
The authors thank to Azuma M, MT. CIAC, Department of Diagnostic Pathology, National Hospital Organization Hakodate Hospital, Hakodate, Japan, for the technical support in immunohistochemistry.
Funding
This study was supported in part by a Grant-in-Aid from the Japan Agency for Medical Research and Development (AMED) under Grant Number 20ek0109352h0003.
Author information
Authors and Affiliations
Contributions
NK and KS designed the study. KS, KK, YO, CS, JS, ST, HA, SS, TY, TK, and MN collected materials and clinical data. KT analyzed gene mutations. NK analyzed pathologic data and interpretation, and wrote the first draft of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Institutional Review Board Statement
The paraffin sections used in this study were obtained from archival materials. Institutional Ethical Board in National Hospital Organization Hakodate Hospital approved the research, #R3-0730001.
Informed Consent Statement
Informed consent for the research was obtained from all patients at each institute.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kimura, N., Shiga, K., Kaneko, Ki. et al. Immunohistochemical Expression of Choline Acetyltransferase and Catecholamine-Synthesizing Enzymes in Head-and-Neck and Thoracoabdominal Paragangliomas and Pheochromocytomas. Endocr Pathol 32, 442–451 (2021). https://doi.org/10.1007/s12022-021-09694-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-021-09694-x