Abstract
Background
In patients with iodine-negative thyroid cancer (TC), current guidelines endorse an [18F]FDG PET/CT to identify dedifferentiated sites of disease. We aimed to determine the rate of oncological management changes triggered by such a molecular imaging approach, along with the impact on outcome.
Methods
42 consecutive patients with negative findings on [131I] whole body scan were scheduled for [18F]FDG PET/CT and treatment based on PET results were initiated. To determine the impact on oncological management, we compared the therapeutic plan prior to and after molecular imaging. Based on imaging follow-up, the rate of controlled disease (CD, defined as stable disease, complete or partial response) was also recorded, thereby allowing to assess whether [18F]FDG-triggered management changes can also lead to favorable outcome.
Results
We observed no alterations of the treatment plan in 9/42 (21.4%) subjects (active surveillance in 9/9 [100%]). Oncological management was changed in the remaining 33/42 (78.6%; systemic treatment in 9/33 [27.3%] and non-systemic treatment in 24/33 [72.7%]). Among patients receiving non-systemic therapy, the following changes were noted: surgery in 20/24 (83.3%) and radiation therapy in 4/24 (16.7%). In the systemic group, tyrosine kinase inhibitor (TKI) was prescribed in 8/9 (88.9%), while radioiodine therapy based on a TKI-mediated redifferentiation approach was conducted in 1/9 (11.1%). In 26 subjects with available follow-up, rate of CD was 22/26 (84.6%) and among those, 15/22 (68.1%) had experienced previous management changes based on PET/CT findings.
Conclusions
In subjects with iodine-negative TC, [18F]FDG PET/CT triggered relevant management changes along with disease control in the vast majority of patients. As such, in dedifferentiated TC, [18F]FDG PET/CT may serve as a relevant management tool and therapeutic decision-aid in the clinic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current guidelines endorse radioiodine therapy (RIT) after thyroidectomy in patients diagnosed with differentiated thyroid cancer (TC) and such a sequential therapeutic approach provides favorable outcome along with complete remission (CR) in most of the patients [1]. A [131I] whole body scan (WBS) may rule out thyroid remnants or widespread metastatic disease. Downregulation of the sodium-iodine symporter, however, may then cause a [131I] loss in target lesions [2], indicative for dedifferentiation of disease. This clinical scenario, however, may then pose a challenge for the treating endocrinologist or nuclear medicine specialist. As such, negative WBS then requires further diagnostic work-up to identify active sites of disease [1]. For instance, conventional imaging including computed tomography (CT) or magnetic resonance imaging (MRI) have yielded mixed results in detecting dedifferentiating lesions [3]. [18F]FDG, however, has also been applied in such patients and has provided excellent read-out capabilities due to increased glucose consumption in TC lesions with [131I] loss [4]. In this regard, a growing body of evidence has already demonstrated the clinical value of a dual-radiotracer strategy of [131I] WBS / [18F]FDG PET/CT. Among others, Palmedo et al. reported on a diagnostic accuracy of more than 90% in this patient population [4], while quantitative parameters derived from [18F]FDG also had prognostic ability for outcome [5, 6]. Given those favorable diagnostic and prognostic performance, a recent study investigated the impact of [18F]FDG on oncological management in iodine-negative TC patients and reported on 10/21 (48%) with altered treatment plan [4]. However, in this previous investigation, the therapeutic armamentarium in dedifferentiated disease did not include tyrosine kinase inhibitor (TKI) or redifferentiation approaches for re-administration of [131I] at that time. Thus, in this previous study, the authors only reported on surgical intervention as management changes, i.e., alterations of the treatment plan were limited to non-systemic therapeutic changes [4].
In the present study, we aimed to determine the impact on oncological management of [18F]FDG PET/CT in iodione-negative TC patients, particularly focusing on systemic treatment alterations (TKI, RIT after iodine uptake restoration) vs. non-systemic changes (surgery, external beam radiation [RTx]).
Material and methods
General
Due to the retrospective character, the local ethics committee at the University Hospital of Würzburg waived the need for further approval (No. 20230721 01). A previous case reported on one subject of this cohort which was scheduled for a redifferentiation approach, i.e., RIT was performed due to restored radioiodine uptake mediated by previous TKI intake [7].
Patient population
42 patients (29 female) between 18 and 85 years (59.7 ± 19.4) with a histologically confirmed TC (27/42 [64.3%] papillary, 11/42 [26.2%] follicular, 3/42 [7.1%] oncocytic, and 1/42 [2.4%] insular TC) were included. All patients had undergone previous surgery and RIT (median cumulative activity, 12 GBq [131I]) and showed no relevant uptake on WBS along with increasing tumor marker thyroglobulin (TG). [18F]FDG PET/CT for restaging was then performed as part of clinical routine follow-up. All subjects included in the study gave informed consent for procedures. A supplementary table provides a detailed overview on previous therapies and TG at time of scan.
Imaging procedures
[18F]FDG PET/CT low dose technique was performed in all patients with a Siemens Biograph mCT 64 or 128 (Siemens Healthineers, Erlangen, Germany) scanner. The imaging procedure was performed 60 min after the injection of approximately 292 ± 41 MBq of [18F]FDG. A scan of the whole body (from the top of the skull to the proximal thigh) was performed. The section thickness of the PET/CT was 5 mm. All PET images were iteratively reconstructed in accordance with the manufacturer’s instructions (Siemens Healthineers, Erlangen, Germany).
Change in oncological management based on [18F]FDG PET/CT
Treatment plans right before and after [18F]FDG PET/CT were retrieved from our medical archive. Treatment alterations were either classified as systemic (TKI [including dose alterations], redifferentation approach using RIT after TKI intake) or non-systemic therapy (surgery, RTx). Surgical approaches were also divided into cervical vs. thoracic procedures.
Impact of management change on disease control
To determine the impact of management change on CD, the first follow-up imaging (conducted within one year) after [18F]FDG PET/CT was assessed. We applied RECIST criteria 1.1. Stable, partial, or complete response was then summarized as CD [8, 9] .
Statistical analysis
Descriptive statistics are indicated as mean ± SD. We used Excel for Windows 2016 (Microsoft, Redmond, WA, USA) and GraphPad Prism Software (9.3.1; La Jolla, CA, USA).
Results
[18F]FDG PET/CT identified sites of disease in all patients
On a patient-based level, [18F]FDG PET/CT was positive in all subjects (100%) and the following disease sites on an organ-based level were recorded: 9/42 (21.4%) patients had a local recurrence, and 24/42 (57.1%) presented with lymph node (LN) metastases [16/24 (66.7%) cervical and 8/24 (33.3%) mediastinal LN]. 21/42 (50%) had lung, 4/42 (9.5%) bone and 1/42 (2.4%) liver metastases (Table 1).
[18F]FDG PET/CT led to change in oncological management in more than 78%
We observed no changes of the treatment plan after [18F]FDG PET/CT in 9/42 (21.4%) subjects and in those individuals, active surveillance was conducted (9/9 [100%]).
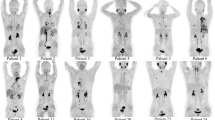
Oncological management was changed in the remaining 33/42 (78.6%). When subdividing those patients into non-systemic vs. systemic therapy, 9/33 (27.3%) were allocated to the latter subgroup. TKI was then prescribed in 8/9 (88.9%), while radioiodine therapy was conducted in 1/9 (11.1%, applying a TKI-based redifferentiation approach). After [18F]FDG, non-systemic treatment was then applied in 24/33 (72.7%) and those included RTx in 4/24 (16.7%) and surgery in 20/24 (83.3%; Fig. 1). Among patients scheduled for operative procedures, cervical surgery was conducted in 12/20 (60.0%) and thoracic surgery in 8/20 (40.0%). Figure 2 provides two cases, in whom [18F]FDG PET/CT led to systemic treatment initiation (TKI, A) and non-systemic management change (cervical LN dissection, B).
Patients with management changes after [18F]FDG PET/CT, with (A) systemic and (B) non-systemic treatment alterations. Maximum intensity projections and transaxial CT and PET/CT portions of target lesions are also displayed. Subject in A showed multiple organ involvement with PET-positive pulmonal and osseous findings (in the left pelvis) displayed on transaxial CT and PET/CT. Of note, lung lesions were only identifiable in the PET/CT images. Additional PET-positive hepatic lesions are not shown on transaxial slides, but seen on the MIP (arrows). Given widespread disease revealed by [18F]FDG, this patient was scheduled for tyrosine kinase inhibitor. Patient in B, however, only presented with cervical PET-positive lymph nodes (arrow, also seen on transaxial PET and PET/CT), triggering neck dissection of [18F]FDG-avid findings. Both patients then presented with controlled disease upon follow-up
[18F]FDG PET/CT-triggered management changes led to disease control in more than 68%
In the entire cohort, follow-up imaging was available in 26/42 (61.9%) and CD was then recorded in 22/26 (84.6%). In this subgroup, 15/22 (68.1%) had experienced previous management changes based on PET findings and in those subjects, the majority (12/15 [80%]) had received non-systemic treatment (surgery in 9/12 [75%] and RTx in 3/12 [25%]). The remaining 3/12 (25%) presenting with CD upon follow-up, however, had received systemic treatment after PET/CT (TKI in 2/3 [66.7%] and RIT in 1/3 [33.3%]). In a supplementary table, we provided a comparison of the pathological findings on cross sectional imaging relative to [18F]FDG PET, which led to respective management changes.
Discussion
In patients with dedifferentiated TC scheduled for [18F]FDG after the first negative WBS, PET/CT led to management changes in more than 78% of the patients. Of those subjects, the majority was allocated to non-systemic alterations, mainly including surgical interventions. After PET/CT, however, approximately one-fourth of the subjects were also treated systemically using TKI (or TKI in combination with RIT). Of note, the [18F]FDG-triggered change in management then also led to disease control in more than 68% of the subjects. Taken together, even with an increasing therapeutic armamentarium in iodine-negative TC patients [10], [18F]FDG PET/CT provided crucial therapeutic decision-aid to identify the most appropriate treatment. Of note, this applied to subjects with limited (scheduled for surgery or RTx) and patients with widespread disease (triggering TKI prescription or TKI-mediated RIT).
Loss of sodium-iodine transporter in TC may pose a challenge, as RIT no longer provides anti-tumor efficacy and thus, in patients with negative WBS with or without rising TG, [18F]FDG may identify dedifferentiating tumor lesions [4]. Previous reports have reported on excellent diagnostic accuracy [4], but information on clinical relevance is rather limited. In this regard, a previous investigation published in 2006 showed that PET/CT can change management in a substantial fraction of patients, mainly surgical interventions. In the last two decades, a plethora of novel therapies have entered the clinical arena [10], including lenvatinib in iodine-refractory TC with response rates of almost 65% when compared to placebo [11]. Moreover, favorable outcome has also been reported using pazopanib or sorafenib [12, 13], which even led to approval by respective agencies [14]. As such, given the beneficial evidence of such oral kinase inhibitors in iodine-refractory subjects in recent years, we aimed to determine whether [18F]FDG is still useful to guide the treating physician towards the most appropriate next therapeutic step beyond surgical procedures. We recorded management alterations in >78% of the subjects, with disease control in 68.1% that had experienced previous changes of the treatment plan. This is slightly increased when compared to the findings of Palmedo et al. (48%) [4] and may be partially explained by the improved scanner technology in our study (Biograph mCT 64 or 128) along with improved read-out. Systemic treatment alterations were rather less frequent in our analysis and included use of TKI, but also redifferentiation approaches, i.e., restoration of radioiodine uptake due to TKI-mediated modulation of the sodium-iodine symporter in TC cells [15]. Due to the increasing use of such sophisticated therapeutic approaches of target re-activation [7, 16], our study also provides a hint that [18F]FDG could serve as a decision-aid to identify patients eligible for such a salvage approach.
Of note, comparable to previous work [4], we also recorded surgery as the most frequent intervention after [18F]FDG and those findings are most likely due to the fact that patients were included right after the first negative WBS (i.e., at an early stage of re-differentiating disease). As such, future studies may also investigate individuals at later stage, which may then also demonstrate that [18F]FDG PET/CT can also trigger a higher rate of systemic therapeutic interventions (such as TKI). Nonetheless, the present and previous studies indicate that this radiotracer is beneficial in subjects presenting with iodine-negative WBS. This applies in particular to limited cervical disease, where [18F]FDG can then precisely localize metastatic LN, thereby guiding the endocrine surgeon or otorhinolaryngologist in pre-operative planning. Of note, a substantial fraction of individuals in our study also presented with thoracic findings revealed by molecular imaging, which then also triggered interventions by thoracic surgery in 40% (including dissection of mediastinal LN and lung metastases). Those findings may be of relevance, as a previous study also reported that loss of radioiodine avidity in pulmonal lesions is an independent predictor of poor outcome, thereby emphasizing the importance of an accurate thoracic read-out by [18F]FDG PET/CT in those high-risk patients [17].
Prospective studies are needed to corroborate our preliminary findings. Future analyses, however, may also consider to repeat our investigation in a larger number of patients, by pooling data from multiple centers or by using other, sensitive PET agents, e.g., [18F]tetrafluoroborate [18, 19] or theranostic agents also used in such a patient population, e.g., prostate-specific membrane antigen-targeted PET/CT [20].
Conclusions
In patients with dedifferentiated TC scheduled for [18F]FDG after the first negative WBS, PET/CT led to management changes in the vast majority of patients, including systemic (TKI, RIT after re-differentiation) and non-systemic treatment changes (thoracic/cervical surgery, RTx). Those alterations of the treatment plan achieved controlled disease in more than 68% of the patients. The high rate of PET/CT-triggered surgical interventions indicate that [18F]FDG may be particularly useful to precisely localize limited cervical or widespread thoracic disease, thereby also serving as a valuable pre-operative planning tool.
References
B.R. Haugen, E.K. Alexander, K.C. Bible, G.M. Doherty, S.J. Mandel, Y.E. Nikiforov et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26(1), 1–133 (2016)
G. Dai, O. Levy, N. Carrasco, Cloning and characterization of the thyroid iodide transporter. Nature 379(6564), 458–460 (1996)
J.K. Hoang, B.F.T. Branstetter, A.R. Gafton, W.K. Lee, C.M. Glastonbury, Imaging of thyroid carcinoma with CT and MRI: approaches to common scenarios. Cancer Imaging 13(1), 128–139 (2013)
H. Palmedo, J. Bucerius, A. Joe, H. Strunk, N. Hortling, S. Meyka et al. Integrated PET/CT in differentiated thyroid cancer: diagnostic accuracy and impact on patient management. J Nucl Med 47(4), 616–624 (2006)
H. Wang, H. Dai, Q. Li, G. Shen, L. Shi, R. Tian, Investigating (18)F-FDG PET/CT parameters as prognostic markers for differentiated thyroid cancer: a systematic review. Front Oncol 11, 648658 (2021)
M. Liu, L. Cheng, Y. Jin, M. Ruan, S. Sheng, L. Chen, Predicting (131)I-avidity of metastases from differentiated thyroid cancer using (18)F-FDG PET/CT in postoperative patients with elevated thyroglobulin. Sci Rep 8(1), 4352 (2018)
R.A. Werner, C. Sayehli, H. Hanscheid, T. Higuchi, S.E. Serfling, M. Fassnacht et al. Successful combination of selpercatinib and radioiodine after pretherapeutic dose estimation in RET-altered thyroid carcinoma. Eur J Nucl Med Mol Imaging 50(6), 1833–1834 (2023)
S.E. Serfling, P.E. Hartrampf, Y. Zhi, T. Higuchi, A. Kosmala, J. Serfling et al. Somatostatin receptor-directed PET/CT for therapeutic decision-making and disease control in patients affected with small cell lung cancer. Clin Nucl Med 48(4), 309–314 (2023)
S.E. Serfling, Y. Zhi, F. Megerle, M. Fassnacht, A.K. Buck, C. Lapa et al. Somatostatin receptor-directed molecular imaging for therapeutic decision-making in patients with medullary thyroid carcinoma. Endocrine 78(1), 169–176 (2022)
T. Fullmer, M.E. Cabanillas, M. Zafereo, Novel therapeutics in radioactive iodine-resistant thyroid cancer. Front Endocrinol 12, 720723 (2021)
M. Schlumberger, M. Tahara, L.J. Wirth, B. Robinson, M.S. Brose, R. Elisei et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372(7), 621–630 (2015)
K.C. Bible, V.J. Suman, J.R. Molina, R.C. Smallridge, W.J. Maples, M.E. Menefee et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol 11(10), 962–972 (2010)
M.S. Brose, C.M. Nutting, B. Jarzab, R. Elisei, S. Siena, L. Bastholt et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384(9940), 319–328 (2014)
L.L. Covell, A.K. Ganti, Treatment of advanced thyroid cancer: role of molecularly targeted therapies. Target Oncol 10(3), 311–324 (2015)
L. Lamartina, N. Anizan, C. Dupuy, S. Leboulleux, M. Schlumberger, Redifferentiation-facilitated radioiodine therapy in thyroid cancer. Endocr Relat Cancer 28(10), T179–T191 (2021)
L. Groussin, L. Bessiene, J. Arrondeau, S. Garinet, B. Cochand-Priollet, A. Lupo et al. Letter to the Editor: selpercatinib-enhanced radioiodine uptake in RET-rearranged thyroid cancer. Thyroid 31(10), 1603–1604 (2021)
Z.L. Qiu, C.T. Shen, Z.K. Sun, H.J. Song, G.Q. Zhang, Q.Y. Luo, Lung metastases from papillary thyroid cancer with persistently negative thyroglobulin and elevated thyroglobulin antibody levels during radioactive iodine treatment and follow-up: long-term outcomes and prognostic indicators. Front Endocrinol 10, 903 (2019)
S. Samnick, E. Al-Momani, J.S. Schmid, A. Mottok, A.K. Buck, C. Lapa, Initial clinical investigation of [18F]Tetrafluoroborate PET/CT in comparison to [124I]Iodine PET/CT for imaging thyroid cancer. Clin Nucl Med 43(3), 162–167 (2018)
M. Dittmann, J.M. Gonzalez Carvalho, K. Rahbar, M. Schafers, M. Claesener, B. Riemann et al. Incremental diagnostic value of [(18)F]tetrafluoroborate PET-CT compared to [(131)I]iodine scintigraphy in recurrent differentiated thyroid cancer. Eur J Nucl Med Mol Imaging 47(11), 2639–2646 (2020)
L.H. de Vries, L. Lodewijk, A. Braat, G.C. Krijger, G.D. Valk, M. Lam et al. 68Ga-PSMA PET/CT in radioactive iodine-refractory differentiated thyroid cancer and first treatment results with (177)Lu-PSMA-617. EJNMMI Res. 10(1), 18 (2020)
Author contributions
Conceptualization: Y.Z., R.A.W. and S.E.S. Methodology: Y.Z., R.A.W. and S.E.S. Formal analysis: Y.Z., R.A.W. and S.E.S. Resources: R.H. Original draft preparation: Y.Z., R.A.W. and S.E.S. Writing—review and editing: T.H., S.H., R.H., M.S. and A.S. All authors have read and agreed to the published version of the manuscript.
Fundings
This study was partially supported by the German Research Foundation (453989101, RAW, TH; 507803309, RAW), Okayama University “RECTOR” Program, and a KAKENHI grant (22H03027) from the Japan Society for the Promotion of Science (both TH). Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhi, Y., Higuchi, T., Hackenberg, S. et al. [18F]FDG PET/CT can trigger relevant oncological management changes leading to favorable outcome in iodine-negative thyroid cancer patients. Endocrine 84, 656–662 (2024). https://doi.org/10.1007/s12020-023-03645-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03645-8