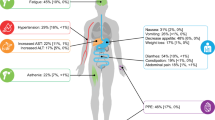
Abstract
Purpose
Multitargeted kinase inhibitors (MKIs) are used for the treatment of several cancers. By targeting multiple signaling pathways, MKIs have become cornerstones of the oncologic treatment. Although their use leads to important results in terms of survival, treatment with MKIs can determine important side effects the clinician must be aware of. Among those, arterial hypertension, mucositis and skin lesions are universally reported, while data about metabolic alterations are scarce. In our review, we focused on glucose and lipid alterations in MKI-treated patients.
Methods
We searched for articles, published between January 2012 and December 2022, evaluating the effects on lipid and glucose metabolism of four MKIs (Cabozantinib, Lenvatinib, Sorafenib, and Vandetanib) in adult patients with cancer. We focused on drugs approved for thyroid malignancies, since a worse metabolic control may potentially impact life expectancy, due to their better overall survival rate.
Results
As for glucose metabolism, the majority of the studies reported elevation of glucose levels (prevalence: 1–17%) with different grades of severity, including death. As for cholesterol, 12 studies reported worsening or new-onset hypercholesterolemia (prevalence: 4–40%). Finally, 19 studies reported different grades of hypertriglyceridemia (prevalence: 1–86%), sometimes leading to life-threatening events.
Conclusions
Despite some inherent limitations, our analysis may cast light upon some of the MKIs metabolic disorders that can impact on patients’ health, especially when long-term survival is expected. Future clinical trials should consider routine assessment of glucose and lipid levels, because underdetection and underreporting of alterations can lead to the overlooking of important adverse events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multitargeted kinase inhibitors (MKI) are increasingly approved and used for multiple solid and hematological malignant neoplasms and target several molecular pathways involved with cellular growth and de-differentiation, such as vascular endothelial growth factor, fibroblast growth factor, platelet-derived growth factor, and c-Kit pathways. These drugs are collectively referred to as antiangiogenic drugs, because they also interfere with angiogenesis, to differentiate them from more recent molecules, designed to be selective to specific mutations or pathways [1].
Due to their off-target activities and interactions with multiple kinases, several systemic side effects are commonly reported with these drugs (e.g., arterial hypertension, mucositis, and skin lesions) [2, 3].
During randomized clinical trials, not all adverse events were reported. As commonly recognized, data from controlled clinical trials are rarely reproducible in real-life practice, due to their highly controlled setting (optimized to show the effect of the drug), and their carefully selected populations, usually not the same to which the drug is prescribed in clinical practice [4].
Furthermore, drug side effects are heterogeneous by nature, being influenced by age, drug interactions, pharmacokinetics and pharmacodynamics, and developing methods to identify patients at higher risk has proved challenging [5]. Their underdetection and consequent underreporting may thus lead to an underestimate of the impact on patients’ health.
For example, some subtle, biochemical side effects of Lenvatinib (adrenal insufficiency [6,7,8], or hypocalcemia) were not specifically reported in the SELECT trial [9], nor in the early real-world reports [10,11,12]. While the metabolic adverse effects of MKIs are known [13, 14], data on their severity and prevalence are scarce. In this review, we focused on the alterations of glucose and lipid metabolism in patients treated with some commonly used MKIs (Cabozantinib, Lenvatinib, Sorafenib, and Vandetanib).
We chose to focus on drugs approved for thyroid malignancies. These patients have a better overall survival rate (53.3% 5-year relative survival rate, even in cases with distant metastases, Table 1), and a worse metabolic control may impact life expectancy.
Methods
The study protocol was registered on PROSPERO (registration number CRD42023387091).
We performed literature research in twelve databases (PubMed, ISI, mRCT, EMBASE, Cochrane, Clinical trial.gov, Scopus, GHL, POPLINE, Google Scholar, VHL and SIGLE).
The research was carried out using the following wording: (cabozantinib OR lenvatinib OR sorafenib OR vandetanib) AND (“metabolic effects” OR “high glucose” OR “low glucose” OR hyperglycemia OR hypoglycemia OR “glucose alterations” OR dyslipidemia OR hyperlipidemia OR hypercholesterolemia OR cholesterol OR “high cholesterol” OR hypertriglyceridemia OR triglycerides OR “high triglycerides” OR “lipid alterations”). Additional articles were searched analyzing the bibliographic references of the selected articles.
We searched for articles evaluating the effects on glucose and lipid metabolism of four MKIs (Cabozantinib, Lenvatinib, Sorafenib and Vandetanib) in adult patients with cancer.
All types of English-language trials and studies evaluating the effect of Cabozantinib, Lenvatinib, Sorafenib and Vandetanib on glucose or lipid metabolism in adult patients were included in our study.
In vitro studies, studies on animals, no full-text articles, case reports and studies on pediatric population were excluded.
Studies published before January 2012 and studies involving a population of <10 patients were subsequently excluded as well (see Fig. 1).
The initial research in PubMed, EMBASE, Cochrane, Scopus and POPLINE provided 15, 98, 37, 333, and 203 studies respectively. After title and abstract screening, respectively 12, 5, 19, 82, and 3 were selected. Research through the other databases did not provide results consistent with our inclusion criteria. After duplicates were removed, 90 studies were selected for full-text screening and 69 studies met the inclusion criteria. One study was subsequently excluded because of the plausible confounding effect of one molecule (Trebananib).
The research was independently carried out by two investigators (EA and CM), using the same searching strategy. Only one difference in study selection was assessed, and discussed to reach consensus, with the opinion of a third author (MM).
The following data were extracted from each article: type of study, publication year, name of the journal, molecule/s analyzed, recruitment time, population (number of patients), sex, median age, underlying pathology, comorbidities, duration of therapy, daily dose, number of patients undergoing permanent treatment interruption due to adverse events and timing of interruption and effect on glucose, cholesterol and triglyceride metabolism (frequency, timing, grade according to Common Terminology Criteria for Adverse Events [CTCAE]). We chose to report on Table 2 and Fig. 2 only those studies reporting effects on glucose of grade > or = to 3 according to the CTCAE classification.
Metabolic effects of multitargeted kinase inhibitors
Molecularly targeted therapy has become one of the cornerstones of personalized medicine in the oncology field and consists of drugs that specifically interfere with dysregulated signaling pathways in neoplastic cells. Currently, a large spectrum of drugs interfering with cancer cells functions or the tumor microenvironment is being developed. Among those, MKIs target common mechanisms of proliferation, local invasion, metastasis and angiogenesis [15].
Presently, MKIs are widely used for treating several classes of malignant disease, both as a single agent or in combination, and their additional mechanisms of action are still not completely understood [16]. When well tolerated, treatment with a MKI can lead to significant results in terms of overall survival but, occasionally, especially when used in long-term protocol treatments, they can also determine important side effects the clinician must be aware of [3]. Among these effects, glucose and lipids serum levels alterations are not always investigated [17].
As for glucose metabolism, many evidences suggest that MKIs can influence glucose levels through different pathways. Most importantly, MKIs belonging to the same class can determine both hyper- or hypoglycemia [18]. Only a small number of reports (3/68) [19,20,21] described glucose-lowering effects associated with MKIs use. On the other hand, a great number of studies (42/68) reported elevation of serum glucose levels in patients treated with MKIs, while neutral effects were reported in a minority of cases (25/68): 18 studies reported only grade 1 or 2 hyperglycemia [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38], while the others reported also CTCAE grades 3 or 4 (Table 2). Interestingly, Sorafenib is frequently associated with all CTCAE grades of hyperglycemia, including death, both as a single agent or in combination with other MKIs (Table 2, Fig. 2). Furthermore, Sorafenib is the only MKI, among those included in our review, known to determine hypoglycemic episodes of different severity, including grade 3 or greater, in patients treated for hepatocellular carcinoma or glioma [19,20,21]. Cabozantinib, Lenvatinib and Vandetanib are mostly associated with mild to moderate high blood glucose (Table 2) and no studies showed evidence of grade 5 hyperglycemia or hypoglycemia associated with their use.
As regards cholesterol metabolism, worsening or new onset of high serum cholesterol levels has been reported in only 12 studies (Table 3, Fig. 3). Most studies (7/12) reported CTCAE grade 3 and 4 hypercholesterolemia, while grade 5 was not reported. Sorafenib, Cabozantinib and Lenvatinib have all been implicated in new onset of different grades of hypercholesterolemia, either when used as a single agent or in combination with other MKIs. Cholesterol metabolism alterations associated with Vandetanib were not described.
Regarding triglycerides metabolism, 19 studies reported on the occurrence of hypertriglyceridemia in patients treated with MKIs. Cabozantinib, Lenvatinib, Sorafenib and Vandetanib adversely affect triglycerides metabolism with different degrees of severity, from mild to life-threatening levels (Fig. 4, Table 4).
Sources of heterogeneity
CTCAE definitions
In the examined literature, toxicities are usually graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) on a 1–5 scale: 1 = mild, 2 = moderate, 3 = severe, 4 = life-threatening with urgent intervention indicated, 5 = death related to adverse events (AEs). Interestingly, the reviewed studies assessed AEs using different CTCAE versions (v3.0, v4.0 and v5.0), that differ mainly in the v5.0 definition of hyperglycemia (Table 5).
Gender
Most of the patients included in the studies were males, which might represent a potential bias, since lipid metabolism is differentially regulated in males and females.
Underreporting
In a minority of papers included in our analysis, toxicities were reported only when graded ≥ 3 according to CTCAE thus leading to important underreporting of mild to moderate adverse events. Furthermore, many studies did not report any glucose or lipid-related AEs, probably due to the lack of planned evaluations in the design of the study. Consistently, this type of AEs are a very small fraction of those reported to the FDA Adverse Event Reporting System (FAERS) (Table 6).
Limitations of the review
This systematic review had some limitations. The examined literature reported studies of different designs (randomized clinical trials, prospective studies and retrospective studies) conducted by different research groups in different countries, so potential biases could not be avoided. In many reports, patients were treated with a combination of drugs (MKI plus at least another antineoplastic agent) so that linking the occurrence of specific AEs to a single molecule was not possible (Table 6). Furthermore, some studies evaluated toxicities of severe grades, thus providing only a partial picture of the phenomena described.
Moreover, information about different lipoprotein lipids concentrations (i.e., HDL cholesterol, LDL cholesterol) are lacking in the selected articles, limiting the understanding of the potential atherogenic risk linked to these metabolic disturbances.
In our opinion, clinicians should be familiar with metabolic disorders that MKI-treated patients could develop, including dysglycemia and dyslipidemia. It is important to periodically evaluate glucose and lipid levels in order to recognize and control these adverse events.
In summary, further investigation is necessary for a more comprehensive understanding of the adverse metabolic profile of MKIs. Underdetection and consequent underreporting of potential alterations can lead to the overlooking of important adverse events, especially in the context of MKI- treated patients with a longer overall survival. Future clinical trials should include in their protocol design routine assessment of glucose and lipid profile in order to allow a better understanding of the prevalence of these alterations, identifying subgroups at risk, and possibly paving the way for discovering new molecular mechanisms responsible for these adverse effects.
References
A. Verrienti, G. Grani, M. Sponziello, V. Pecce, G. Damante, C. Durante, D. Russo, S. Filetti, Precision oncology for RET-related tumors. Front. Oncol. 12, 992636 (2022). https://doi.org/10.3389/fonc.2022.992636
M.E. Cabanillas, S. Takahashi, Managing the adverse events associated with lenvatinib therapy in radioiodine-refractory differentiated thyroid cancer. Semin. Oncol. 46, 57–64 (2019). https://doi.org/10.1053/j.seminoncol.2018.11.004
C. Colombo, S. De Leo, M. Trevisan, N. Giancola, A. Scaltrito, L. Fugazzola, Daily Management of Patients on Multikinase Inhibitors’ Treatment. Front. Oncol. 12, 903532 (2022). https://doi.org/10.3389/fonc.2022.903532
M.G.P. Zuidgeest, I. Goetz, R.H.H. Groenwold, E. Irving, G.J.M.W. van Thiel, D.E. Grobbee, Series: Pragmatic trials and real world evidence: Paper 1. Introduction. J. Clin. Epidemiol. 88, 7–13 (2017). https://doi.org/10.1016/j.jclinepi.2016.12.023
J.M. Stevenson, J.L. Williams, T.G. Burnham, A.T. Prevost, R. Schiff, S.D. Erskine, J.G. Davies, Predicting adverse drug reactions in older adults; a systematic review of the risk prediction models. Clin. Inter. Aging 9, 1581–1593 (2014). https://doi.org/10.2147/CIA.S65475
C. Colombo, S. De Leo, M. Di Stefano, G. Vannucchi, L. Persani, L. Fugazzola, Primary Adrenal Insufficiency During Lenvatinib or Vandetanib and Improvement of Fatigue After Cortisone Acetate Therapy. J. Clin. Endocrinol. Metab. 104, 779–784 (2019). https://doi.org/10.1210/jc.2018-01836
S. Monti, F. Presciuttini, M.G. Deiana, C. Motta, F. Mori, V. Renzelli, A. Stigliano, V. Toscano, G. Pugliese, M. Poggi, Cortisol Deficiency in Lenvatinib Treatment of Thyroid Cancer: An Underestimated Common Adverse Event. Thyroid. Thy. 2021, 0040 (2021). https://doi.org/10.1089/thy.2021.0040
E. Raschi, M. Fusaroli, V. Giunchi, A. Repaci, C. Pelusi, V. Mollica, F. Massari, A. Ardizzoni, E. Poluzzi, U. Pagotto, G. Di Dalmazi, Adrenal Insufficiency with Anticancer Tyrosine Kinase Inhibitors Targeting Vascular Endothelial Growth Factor Receptor: Analysis of the FDA Adverse Event Reporting System. Cancers (Basel) 14, 4610 (2022). https://doi.org/10.3390/cancers14194610
M. Schlumberger, M. Tahara, L.J. Wirth, B. Robinson, M.S. Brose, R. Elisei, M.A. Habra, K. Newbold, M.H. Shah, A.O. Hoff, A.G. Gianoukakis, N. Kiyota, M.H. Taylor, S.-B. Kim, M.K. Krzyzanowska, C.E. Dutcus, B. de las Heras, J. Zhu, S.I. Sherman, Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 372, 621–630 (2015). https://doi.org/10.1056/NEJMoa1406470
A. Berdelou, I. Borget, Y. Godbert, T. Nguyen, M.-E. Garcia, C.N. Chougnet, A. Ferru, C. Buffet, O. Chabre, O. Huillard, S. Leboulleux, M. Schlumberger, Lenvatinib for the Treatment of Radioiodine-Refractory Thyroid Cancer in Real-Life Practice. Thyroid 28, 72–78 (2018). https://doi.org/10.1089/thy.2017.0205
L.D. Locati, A. Piovesan, C. Durante, M. Bregni, M.G. Castagna, S. Zovato, M. Giusti, T. Ibrahim, E. Puxeddu, G. Fedele, G. Pellegriti, G. Rinaldi, D. Giuffrida, F. Verderame, F. Bertolini, C. Bergamini, A. Nervo, G. Grani, S. Rizzati, S. Morelli, I. Puliafito, R. Elisei, Real-world efficacy and safety of lenvatinib: data from a compassionate use in the treatment of radioactive iodine-refractory differentiated thyroid cancer patients in Italy. Eur. J. Cancer 118, 35–40 (2019). https://doi.org/10.1016/j.ejca.2019.05.031
C. Giani, L. Valerio, A. Bongiovanni, C. Durante, G. Grani, T. Ibrahim, S. Mariotti, M. Massa, F. Pani, G. Pellegriti, T. Porcelli, D. Salvatore, M. Tavarelli, M. Torlontano, L. Locati, E. Molinaro, R. Elisei, Safety and Quality-of-Life Data from an Italian Expanded Access Program of Lenvatinib for Treatment of Thyroid Cancer. Thyroid 31, 224–232 (2021). https://doi.org/10.1089/thy.2020.0276
P. Buffier, B. Bouillet, S. Smati, F. Archambeaud, B. Cariou, B. Verges, Expert opinion on the metabolic complications of new anticancer therapies: Tyrosine kinase inhibitors. Annales d’Endocrinologie 79, 574–582 (2018). https://doi.org/10.1016/j.ando.2018.07.011
P. Fallahi, S.M. Ferrari, G. Elia, F. Ragusa, S.R. Paparo, S. Camastra, V. Mazzi, M. Miccoli, S. Benvenga, A. Antonelli, THERAPY OF ENDOCRINE DISEASE: Endocrine-metabolic effects of treatment with multikinase inhibitors. Eur. J. Endocrinol. 184, R29–R40 (2021). https://doi.org/10.1530/EJE-20-0683
P. Fallahi, V. Mazzi, R. Vita, S. Ferrari, G. Materazzi, D. Galleri, S. Benvenga, P. Miccoli, A. Antonelli, New Therapies for Dedifferentiated Papillary Thyroid Cancer. IJMS 16, 6153–6182 (2015). https://doi.org/10.3390/ijms16036153
C.M. Croce, Oncogenes and Cancer. N. Engl. J. Med. 358, 502–511 (2008). https://doi.org/10.1056/NEJMra072367
F. Castinetti, F. Albarel, F. Archambeaud, J. Bertherat, B. Bouillet, P. Buffier, C. Briet, B. Cariou, P. Caron, O. Chabre, P. Chanson, C. Cortet, C. Do Cao, D. Drui, M. Haissaguerre, S. Hescot, F. Illouz, E. Kuhn, N. Lahlou, E. Merlen, V. Raverot, S. Smati, B. Verges, F. Borson-Chazot, Endocrine side-effects of new anticancer therapies: Overall monitoring and conclusions. Annales d’Endocrinologie 79, 591–595 (2018). https://doi.org/10.1016/j.ando.2018.07.005
J. Villadolid, J.L. Ersek, M.K. Fong, L. Sirianno, E.S. Story, Management of hyperglycemia from epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) targeting T790M-mediated resistance. Transl Lung Cancer Res. 4, 576–583 (2015). https://doi.org/10.3978/j.issn.2218-6751.2015.10.01
P. Savvides, G. Nagaiah, P. Lavertu, P. Fu, J.J. Wright, R. Chapman, J. Wasman, A. Dowlati, S.C. Remick, Phase II Trial of Sorafenib in Patients with Advanced Anaplastic Carcinoma of the Thyroid. Thyroid 23, 600–604 (2013). https://doi.org/10.1089/thy.2012.0103
J.M. Davies, N.S. Dhruva, C.M. Walko, M.A. Socinski, S. Bernard, D.N. Hayes, W.Y. Kim, A. Ivanova, K. Keller, L.R. Hilbun, M. Chiu, E.C. Dees, T.E. Stinchcombe, A phase I trial of sorafenib combined with cisplatin/etoposide or carboplatin/pemetrexed in refractory solid tumor patients. Lung Cancer 71, 151–155 (2011). https://doi.org/10.1016/j.lungcan.2010.05.022
R.B. Den, M. Kamrava, Z. Sheng, M. Werner-Wasik, E. Dougherty, M. Marinucchi, Y.R. Lawrence, S. Hegarty, T. Hyslop, D.W. Andrews, J. Glass, D.P. Friedman, M.R. Green, K. Camphausen, A.P. Dicker, A Phase I Study of the Combination of Sorafenib With Temozolomide and Radiation Therapy for the Treatment of Primary and Recurrent High-Grade Gliomas. Int. J. Radiat. Oncol.*Biol.*Phys. 85, 321–328 (2013). https://doi.org/10.1016/j.ijrobp.2012.04.017
E.L. Mayer, S.J. Isakoff, G. Klement, S.R. Downing, W.Y. Chen, K. Hannagan, R. Gelman, E.P. Winer, H.J. Burstein, Combination antiangiogenic therapy in advanced breast cancer: a phase 1 trial of vandetanib, a VEGFR inhibitor, and metronomic chemotherapy, with correlative platelet proteomics. Breast Cancer Res. Treat. 136, 169–178 (2012). https://doi.org/10.1007/s10549-012-2256-5
C. Nabhan, D. Villines, T.V. Valdez, K. Tolzien, T.M. Lestingi, J.D. Bitran, S.M. Christner, M.J. Egorin, J.H. Beumer, Phase I study investigating the safety and feasibility of combining imatinib mesylate (Gleevec) with sorafenib in patients with refractory castration-resistant prostate cancer. Br. J. Cancer 107, 592–597 (2012). https://doi.org/10.1038/bjc.2012.312
R.K. Kelley, H.S. Nimeiri, P.N. Munster, M.T. Vergo, Y. Huang, C.-M. Li, J. Hwang, M.F. Mulcahy, B.M. Yeh, P. Kuhn, M.S. Luttgen, J.A. Grabowsky, L. Stucky-Marshall, W.M. Korn, A.H. Ko, E.K. Bergsland, A.B. Benson, A.P. Venook, Temsirolimus combined with sorafenib in hepatocellular carcinoma: a phase I dose-finding trial with pharmacokinetic and biomarker correlates. Ann. Oncol. 24, 1900–1907 (2013). https://doi.org/10.1093/annonc/mdt109
J.M. Meyer, K.S. Perlewitz, J.B. Hayden, Y.-C. Doung, A.Y. Hung, J.T. Vetto, R.F. Pommier, A. Mansoor, B.R. Beckett, A. Tudorica, M. Mori, M.L. Holtorf, A. Afzal, W.J. Woodward, E.T. Rodler, R.L. Jones, W. Huang, C.W. Ryan, Phase I trial of preoperative chemoradiation plus sorafenib for high-risk extremity soft tissue sarcomas with dynamic contrast-enhanced MRI correlates. Clin. Cancer Res. 19, 6902–6911 (2013). https://doi.org/10.1158/1078-0432.CCR-13-1594
A. Schwandt, V.E. von Gruenigen, R.M. Wenham, H. Frasure, S. Eaton, N. Fusco, P. Fu, J.J. Wright, A. Dowlati, S. Waggoner, Randomized phase II trial of sorafenib alone or in combination with carboplatin/paclitaxel in women with recurrent platinum sensitive epithelial ovarian, peritoneal, or fallopian tube cancer. Investig. N. Drugs 32, 729–738 (2014). https://doi.org/10.1007/s10637-014-0078-5
M.G. Chheda, P.Y. Wen, F.H. Hochberg, A.S. Chi, J. Drappatz, A.F. Eichler, D. Yang, R. Beroukhim, A.D. Norden, E.R. Gerstner, R.A. Betensky, T.T. Batchelor, Vandetanib plus sirolimus in adults with recurrent glioblastoma: results of a phase I and dose expansion cohort study. J. Neurooncol. 121, 627–634 (2015). https://doi.org/10.1007/s11060-014-1680-2
J.M. Hubbard, G. Kim, M.J. Borad, E. Johnson, R. Qin, J. Lensing, S. Puttabasavaiah, J. Wright, C. Erlichman, A. Grothey, Phase I trial of FOLFIRI in combination with sorafenib and bevacizumab in patients with advanced gastrointestinal malignancies. Investig. N. Drugs 34, 96–103 (2016). https://doi.org/10.1007/s10637-015-0308-5
N. Lainez, J. García-Donas, E. Esteban, J. Puente, M.I. Sáez, E. Gallardo, Á. Pinto-Marín, S. Vázquez-Estévez, L. León, I. García-Carbonero, C. Suárez-Rodríguez, C. Molins, M.A. Climent-Duran, M. Lázaro-Quintela, A. González Del Alba, M.J. Méndez-Vidal, I. Chirivella, F.J. Afonso, M. López-Brea, N. Sala-González, M. Domenech, L. Basterretxea, C. Santander-Lobera, I. Gil-Arnáiz, O. Fernández, C. Caballero-Díaz, B. Mellado, D. Marrupe, J. García-Sánchez, R. Sánchez-Escribano, E. Fernández Parra, J.C. Villa Guzmán, E. Martínez-Ortega, M. Belén González, M. Morán, B. Suarez-Paniagua, M.J. Lecumberri, D. Castellano, Impact on clinical practice of the implementation of guidelines for the toxicity management of targeted therapies in kidney cancer. Prot.-2 Study BMC Cancer 16, 135 (2016). https://doi.org/10.1186/s12885-016-2084-9
G.K. Abou-Alfa, C.-J. Yen, C.-H. Hsu, J. O’Donoghue, V. Beylergil, S. Ruan, N. Pandit-Taskar, B. Gansukh, S.K. Lyashchenko, J. Ma, P. Wan, Y.-Y. Shao, Z.-Z. Lin, C. Frenette, B. O’Neil, L. Schwartz, P.M. Smith-Jones, T. Ohtomo, T. Tanaka, H. Morikawa, Y. Maki, N. Ohishi, Y.-C. Chen, T. Agajanov, F. Boisserie, L. Di Laurenzio, R. Lee, S.M. Larson, A.-L. Cheng, J.A. Carrasquilo, Phase Ib study of codrituzumab in combination with sorafenib in patients with non-curable advanced hepatocellular carcinoma (HCC). Cancer Chemother. Pharm. 79, 421–429 (2017). https://doi.org/10.1007/s00280-017-3241-9
M.E. Cabanillas, J.A. de Souza, S. Geyer, L.J. Wirth, M.E. Menefee, S.V. Liu, K. Shah, J. Wright, M.H. Shah, Cabozantinib As Salvage Therapy for Patients With Tyrosine Kinase Inhibitor-Refractory Differentiated Thyroid Cancer: Results of a Multicenter Phase II International Thyroid Oncology Group Trial. J. Clin. Oncol. 35, 3315–3321 (2017). https://doi.org/10.1200/JCO.2017.73.0226
L. Goyal, H. Zheng, M.B. Yurgelun, T.A. Abrams, J.N. Allen, J.M. Cleary, M. Knowles, E. Regan, A. Reardon, A. Khachatryan, R.K. Jain, V. Nardi, D.R. Borger, D.G. Duda, A.X. Zhu, A phase 2 and biomarker study of cabozantinib in patients with advanced cholangiocarcinoma. Cancer 123, 1979–1988 (2017). https://doi.org/10.1002/cncr.30571
T.K. Choueiri, C. Hessel, S. Halabi, B. Sanford, M.D. Michaelson, O. Hahn, M. Walsh, T. Olencki, J. Picus, E.J. Small, S. Dakhil, D.R. Feldman, M. Mangeshkar, C. Scheffold, D. George, M.J. Morris, Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update. Eur. J. Cancer 94, 115–125 (2018). https://doi.org/10.1016/j.ejca.2018.02.012
A.B. El-Khoueiry, R. O’Donnell, T.J. Semrad, P. Mack, S. Blanchard, N. Bahary, Y. Jiang, Y. Yen, J. Wright, H. Chen, H.-J. Lenz, D.R. Gandara, A phase I trial of escalating doses of cixutumumab (IMC-A12) and sorafenib in the treatment of advanced hepatocellular carcinoma. Cancer Chemother. Pharm. 81, 957–963 (2018). https://doi.org/10.1007/s00280-018-3553-4
A.T. Fathi, T.M. Blonquist, D. Hernandez, P.C. Amrein, K.K. Ballen, M. McMasters, D.E. Avigan, R. Joyce, E.K. Logan, G. Hobbs, A.M. Brunner, C. Joseph, A.M. Perry, M. Burke, T. Behnan, J. Foster, M.K. Bergeron, J.A. Moran, A.Y. Ramos, T.T. Som, J. Rae, K.M. Fishman, K.L. McGregor, C. Connolly, D.S. Neuberg, M.J. Levis, Cabozantinib is well tolerated in acute myeloid leukemia and effectively inhibits the resistance-conferring FLT3/tyrosine kinase domain/F691 mutation. Cancer 124, 306–314 (2018). https://doi.org/10.1002/cncr.31038
M. Ikeda, M. Morimoto, M. Tajimi, K. Inoue, K.A. Benhadji, M.M.F. Lahn, D. Sakai, A phase 1b study of transforming growth factor-beta receptor I inhibitor galunisertib in combination with sorafenib in Japanese patients with unresectable hepatocellular carcinoma. Investig. N. Drugs 37, 118–126 (2019). https://doi.org/10.1007/s10637-018-0636-3
F. Yang, J. Yang, W. Xiang, B.-Y. Zhong, W.-C. Li, J. Shen, S. Zhang, Y. Yin, H.-P. Sun, W.-S. Wang, X.-L. Zhu, Safety and Efficacy of Transarterial Chemoembolization Combined With Immune Checkpoint Inhibitors and Tyrosine Kinase Inhibitors for Hepatocellular Carcinoma. Front. Oncol. 11, 657512 (2021). https://doi.org/10.3389/fonc.2021.657512
M. Cai, W. Huang, J. Huang, W. Shi, Y. Guo, L. Liang, J. Zhou, L. Lin, B. Cao, Y. Chen, J. Zhou, K. Zhu, Transarterial Chemoembolization Combined With Lenvatinib Plus PD-1 Inhibitor for Advanced Hepatocellular Carcinoma: A Retrospective Cohort Study. Front. Immunol. 13, 848387 (2022). https://doi.org/10.3389/fimmu.2022.848387
K.A. Margolin, J. Moon, L.E. Flaherty, C.D. Lao, W.L. Akerley, M. Othus, J.A. Sosman, J.M. Kirkwood, V.K. Sondak, Randomized Phase II Trial of Sorafenib with Temsirolimus or Tipifarnib in Untreated Metastatic Melanoma (S0438. Clin. Cancer Res. 18, 1129–1137 (2012). https://doi.org/10.1158/1078-0432.CCR-11-2488
K.T. Flaherty, S.J. Lee, F. Zhao, L.M. Schuchter, L. Flaherty, R. Kefford, M.B. Atkins, P. Leming, J.M. Kirkwood, Phase III Trial of Carboplatin and Paclitaxel With or Without Sorafenib in Metastatic Melanoma. JCO 31, 373–379 (2013). https://doi.org/10.1200/JCO.2012.42.1529
K.T. Flaherty, J.B. Manola, M. Pins, D.F. McDermott, M.B. Atkins, J.J. Dutcher, D.J. George, K.A. Margolin, R.S. DiPaola, BEST: A Randomized Phase II Study of Vascular Endothelial Growth Factor, RAF Kinase, and Mammalian Target of Rapamycin Combination Targeted Therapy With Bevacizumab, Sorafenib, and Temsirolimus in Advanced Renal Cell Carcinoma—A Trial of the ECOG–ACRIN Cancer Research Group (E2804). JCO 33, 2384–2391 (2015). https://doi.org/10.1200/JCO.2015.60.9727
E.Q. Lee, T.J. Kaley, D.G. Duda, D. Schiff, A.B. Lassman, E.T. Wong, T. Mikkelsen, B.W. Purow, A. Muzikansky, M. Ancukiewicz, J.T. Huse, S. Ramkissoon, J. Drappatz, A.D. Norden, R. Beroukhim, S.E. Weiss, B.M. Alexander, C.S. McCluskey, M. Gerard, K.H. Smith, R.K. Jain, T.T. Batchelor, K.L. Ligon, P.Y. Wen, A Multicenter, Phase II, Randomized, Noncomparative Clinical Trial of Radiation and Temozolomide with or without Vandetanib in Newly Diagnosed Glioblastoma Patients. Clin. Cancer Res. 21, 3610–3618 (2015). https://doi.org/10.1158/1078-0432.CCR-14-3220
T.K. Choueiri, B. Escudier, T. Powles, P.N. Mainwaring, B.I. Rini, F. Donskov, H. Hammers, T.E. Hutson, J.-L. Lee, K. Peltola, B.J. Roth, G.A. Bjarnason, L. Géczi, B. Keam, P. Maroto, D.Y.C. Heng, M. Schmidinger, P.W. Kantoff, A. Borgman-Hagey, C. Hessel, C. Scheffold, G.M. Schwab, N.M. Tannir, R.J. Motzer, Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 373, 1814–1823 (2015). https://doi.org/10.1056/NEJMoa1510016
D. Koeberle, J.-F. Dufour, G. Demeter, Q. Li, K. Ribi, P. Samaras, P. Saletti, A.D. Roth, D. Horber, M. Buehlmann, A.D. Wagner, M. Montemurro, G. Lakatos, J. Feilchenfeldt, M. Peck-Radosavljevic, D. Rauch, B. Tschanz, G. Bodoky, Sorafenib with or without everolimus in patients with advanced hepatocellular carcinoma (HCC): a randomized multicenter, multinational phase II trial (SAKK 77/08 and SASL 29. Ann. Oncol. 27, 856–861 (2016). https://doi.org/10.1093/annonc/mdw054
D.R. Spigel, M.S. Rubin, V.G. Gian, D.L. Shipley, H.A. Burris, R.A. Kosloff, K.C. Shih, R. Quinn, F.A. Greco, J.D. Hainsworth, Sorafenib and continued erlotinib or sorafenib alone in patients with advanced non-small cell lung cancer progressing on erlotinib: A randomized phase II study of the Sarah Cannon Research Institute (SCRI). Lung Cancer 113, 79–84 (2017). https://doi.org/10.1016/j.lungcan.2017.09.007
G. Middleton, D.H. Palmer, W. Greenhalf, P. Ghaneh, R. Jackson, T. Cox, A. Evans, V.E. Shaw, J. Wadsley, J.W. Valle, D. Propper, H. Wasan, S. Falk, D. Cunningham, F. Coxon, P. Ross, S. Madhusudan, N. Wadd, P. Corrie, T. Hickish, E. Costello, F. Campbell, C. Rawcliffe, J.P. Neoptolemos, Vandetanib plus gemcitabine versus placebo plus gemcitabine in locally advanced or metastatic pancreatic carcinoma (ViP): a prospective, randomised, double-blind, multicentre phase 2 trial. Lancet Oncol. 18, 486–499 (2017). https://doi.org/10.1016/S1470-2045(17)30084-0
R.E. Sanborn, J.D. Patel, G.A. Masters, N. Jayaram, A. Stephens, M. Guarino, J. Misleh, J. Wu, N. Hanna, A randomized, double-blind, phase 2 trial of platinum therapy plus etoposide with or without concurrent vandetanib (ZD6474) in patients with previously untreated extensive-stage small cell lung cancer: Hoosier Cancer Research Network LUN06-113: Platinum/Etoposide ± Vandetanib for SCLC. Cancer 123, 303–311 (2017). https://doi.org/10.1002/cncr.30287
M.M. Gounder, M.R. Mahoney, B.A. Van Tine, V. Ravi, S. Attia, H.A. Deshpande, A.A. Gupta, M.M. Milhem, R.M. Conry, S. Movva, M.J. Pishvaian, R.F. Riedel, T. Sabagh, W.D. Tap, N. Horvat, E. Basch, L.H. Schwartz, R.G. Maki, N.P. Agaram, R.A. Lefkowitz, Y. Mazaheri, R. Yamashita, J.J. Wright, A.C. Dueck, G.K. Schwartz, Sorafenib for Advanced and Refractory Desmoid Tumors. N. Engl. J. Med. 379, 2417–2428 (2018). https://doi.org/10.1056/NEJMoa1805052
R. Jones, S. Crabb, J. Chester, T. Elliott, R. Huddart, A. Birtle, L. Evans, J. Lester, S. Jagdev, A. Casbard, C. Huang, T. Madden, G. Griffiths, A randomised Phase II trial of carboplatin and gemcitabine ± vandetanib in first‐line treatment of patients with advanced urothelial cell cancer not suitable to receive cisplatin. BJU Int. 126, 292–299 (2020). https://doi.org/10.1111/bju.15096
C. Gomez-Martin, J. Bustamante, J.F. Castroagudin, M. Salcedo, E. Garralda, M. Testillano, I. Herrero, A. Matilla, B. Sangro, Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 18, 45–52 (2012). https://doi.org/10.1002/lt.22434
J.A. Chan, R.J. Mayer, N. Jackson, P. Malinowski, E. Regan, M.H. Kulke, Phase I study of sorafenib in combination with everolimus (RAD001) in patients with advanced neuroendocrine tumors. Cancer Chemother. Pharm. 71, 1241–1246 (2013). https://doi.org/10.1007/s00280-013-2118-9
J.F. Gibson, F. Foss, D. Cooper, S. Seropian, D. Irizarry, L. Barbarotta, F. Lansigan, Pilot study of sorafenib in relapsed or refractory peripheral and cutaneous T-cell lymphoma. Br. J. Haematol. 167, 141–144 (2014). https://doi.org/10.1111/bjh.12944
E.J. Sherman, L.A. Dunn, A.L. Ho, S.S. Baxi, R.A. Ghossein, M.G. Fury, S. Haque, C.S. Sima, G. Cullen, J.A. Fagin, D.G. Pfister, Phase 2 study evaluating the combination of sorafenib and temsirolimus in the treatment of radioactive iodine-refractory thyroid cancer: Sorafenib/Temsirolimus Thyroid Cancer. Cancer 123, 4114–4121 (2017). https://doi.org/10.1002/cncr.30861
A.G. Duffy, C. Ma, S.V. Ulahannan, O.E. Rahma, O. Makarova-Rusher, L. Cao, Y. Yu, D.E. Kleiner, J. Trepel, M.-J. Lee, Y. Tomita, S.M. Steinberg, T. Heller, B. Turkbey, P.L. Choyke, C.J. Peer, W.D. Figg, B.J. Wood, T.F. Greten, Phase I and Preliminary Phase II Study of TRC105 in Combination with Sorafenib in Hepatocellular Carcinoma. Clin. Cancer Res. 23, 4633–4641 (2017). https://doi.org/10.1158/1078-0432.CCR-16-3171
E. Suzuki, S. Kaneko, T. Okusaka, M. Ikeda, K. Yamaguchi, R. Sugimoto, T. Aramaki, A. Asagi, K. Yasui, K. Sano, A. Hosokawa, N. Kato, H. Ishii, T. Sato, J. Furuse, A multicenter Phase II study of sorafenib in Japanese patients with advanced hepatocellular carcinoma and Child Pugh A and B class. Jpn. J. Clin. Oncol. 48, 317–321 (2018). https://doi.org/10.1093/jjco/hyy010
D. Schiff, K.A. Jaeckle, S.K. Anderson, E. Galanis, C. Giannini, J.C. Buckner, P. Stella, P.J. Flynn, B.J. Erickson, J.F. Schwerkoske, V. Kaluza, E. Twohy, J. Dancey, J. Wright, J.N. Sarkaria, Phase 1/2 trial of temsirolimus and sorafenib in the treatment of patients with recurrent glioblastoma: North Central Cancer Treatment Group Study/Alliance N0572: Temsirolimus/Sorafenib for Recurrent GBM. Cancer 124, 1455–1463 (2018). https://doi.org/10.1002/cncr.31219
L. Goyal, H. Zheng, T.A. Abrams, R. Miksad, A.J. Bullock, J.N. Allen, M.B. Yurgelun, J.W. Clark, A. Kambadakone, A. Muzikansky, M. Knowles, A. Galway, A.J. Afflitto, C.F. Dinicola, E. Regan, T. Hato, E. Mamessier, K. Shigeta, R.K. Jain, D.G. Duda, A.X. Zhu, A Phase II and Biomarker Study of Sorafenib Combined with Modified FOLFOX in Patients with Advanced Hepatocellular Carcinoma. Clin. Cancer Res. 25, 80–89 (2019). https://doi.org/10.1158/1078-0432.CCR-18-0847
I. El Dika, M. Capanu, J.F. Chou, J.J. Harding, M. Ly, A.D. Hrabovsky, R.K.G. Do, J. Shia, B. Millang, J. Ma, E.M. O’Reilly, G.K. Abou‐Alfa, Phase II trial of sorafenib and doxorubicin in patients with advanced hepatocellular carcinoma after disease progression on sorafenib. Cancer Med. 9, 7453–7459 (2020). https://doi.org/10.1002/cam4.3389
R.K. Kelley, N.M. Joseph, H.S. Nimeiri, J. Hwang, L.M. Kulik, Z. Ngo, S.C. Behr, C. Onodera, K. Zhang, A.G. Bocobo, A.B. Benson, A.P. Venook, J.D. Gordan, Phase II Trial of the Combination of Temsirolimus and Sorafenib in Advanced Hepatocellular Carcinoma with Tumor Mutation Profiling. Liver Cancer 10, 561–571 (2021). https://doi.org/10.1159/000518297
C.-H. Lee, A.Y. Shah, D. Rasco, A. Rao, M.H. Taylor, C. Di Simone, J.J. Hsieh, A. Pinto, D.R. Shaffer, R. Girones Sarrio, A.L. Cohn, N.J. Vogelzang, M.A. Bilen, S. Gunnestad Ribe, M. Goksel, Ø.K. Tennøe, D. Richards, R.F. Sweis, J. Courtright, D. Heinrich, S. Jain, J. Wu, E.V. Schmidt, R.F. Perini, P. Kubiak, C.E. Okpara, A.D. Smith, R.J. Motzer, Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): a phase 1b/2 study. Lancet Oncol. 22, 946–958 (2021). https://doi.org/10.1016/S1470-2045(21)00241-2
T.E. Hutson, B. Escudier, E. Esteban, G.A. Bjarnason, H.Y. Lim, K.B. Pittman, P. Senico, A. Niethammer, D.R. Lu, S. Hariharan, R.J. Motzer, Randomized Phase III Trial of Temsirolimus Versus Sorafenib As Second-Line Therapy After Sunitinib in Patients With Metastatic Renal Cell Carcinoma. JCO 32, 760–767 (2014). https://doi.org/10.1200/JCO.2013.50.3961
P. Dürr, K. Schlichtig, C. Kelz, B. Deutsch, R. Maas, M.J. Eckart, J. Wilke, H. Wagner, K. Wolff, C. Preuß, V. Brückl, N. Meidenbauer, C. Staerk, A. Mayr, R. Fietkau, P.J. Goebell, F. Kunath, M.W. Beckmann, A. Mackensen, M.F. Neurath, M. Pavel, F. Dörje, M.F. Fromm, The Randomized AMBORA Trial: Impact of Pharmacological/Pharmaceutical Care on Medication Safety and Patient-Reported Outcomes During Treatment With New Oral Anticancer Agents. JCO 39, 1983–1994 (2021). https://doi.org/10.1200/JCO.20.03088
S.K. Pal, J. Puente, D.Y.C. Heng, H. Glen, P. Koralewski, D. Stroyakovskiy, B. Alekseev, F. Parnis, D. Castellano, T. Ciuleanu, J.L. Lee, K. Sunela, K. O’Hara, T.A. Binder, L. Peng, A.D. Smith, S.Y. Rha, Assessing the Safety and Efficacy of Two Starting Doses of Lenvatinib Plus Everolimus in Patients with Renal Cell Carcinoma: A Randomized Phase 2 Trial. Eur. Urol. 82, 283–292 (2022). https://doi.org/10.1016/j.eururo.2021.12.024
X. Zhang, H. Zhang, J. Dai, Z. Liu, X. Zhu, Y. Ni, X. Yin, G. Sun, S. Zhu, J. Chen, J. Zhao, J. Wang, H. Zeng, P. Shen, The influence of dynamic changes in lipid metabolism on survival outcomes in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors. Jpn. J. Clin. Oncol. 50, 1454–1463 (2020). https://doi.org/10.1093/jjco/hyaa120
A.M. Molina, T.E. Hutson, J. Larkin, A.M. Gold, K. Wood, D. Carter, R. Motzer, M.D. Michaelson, A phase 1b clinical trial of the multi-targeted tyrosine kinase inhibitor lenvatinib (E7080) in combination with everolimus for treatment of metastatic renal cell carcinoma (RCC. Cancer Chemother. Pharmacol. 73, 181–189 (2014). https://doi.org/10.1007/s00280-013-2339-y
G. Grignani, E. Palmerini, V. Ferraresi, L. D’Ambrosio, R. Bertulli, S.D. Asaftei, A. Tamburini, Y. Pignochino, D. Sangiolo, E. Marchesi, F. Capozzi, R. Biagini, M. Gambarotti, F. Fagioli, P.G. Casali, P. Picci, S. Ferrari, M. Aglietta, Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: a non-randomised phase 2 clinical trial. Lancet Oncol. 16, 98–107 (2015). https://doi.org/10.1016/S1470-2045(14)71136-2
R.J. Motzer, C. Porta, N.J. Vogelzang, C.N. Sternberg, C. Szczylik, J. Zolnierek, C. Kollmannsberger, S.Y. Rha, G.A. Bjarnason, B. Melichar, U. De Giorgi, V. Grünwald, I.D. Davis, J.-L. Lee, E. Esteban, G. Urbanowitz, C. Cai, M. Squires, M. Marker, M.M. Shi, B. Escudier, Dovitinib versus sorafenib for third-line targeted treatment of patients with metastatic renal cell carcinoma: an open-label, randomised phase 3 trial. Lancet Oncol. 15, 286–296 (2014). https://doi.org/10.1016/S1470-2045(14)70030-0
K. Pazaitou-Panayiotou, A. Chrisoulidou, S. Mandanas, L. Mathiopoulou, M. Boudina, E. Margaritidou, K. Georgopoulos, Treatment compliance and severe adverse events limit the use of tyrosine kinase inhibitors in refractory thyroid cancer. OTT. 2435 (2015). https://doi.org/10.2147/OTT.S86322
M.A. Davies, P.S. Fox, N.E. Papadopoulos, A.Y. Bedikian, W.-J. Hwu, A.J. Lazar, V.G. Prieto, K.S. Culotta, T.L. Madden, Q. Xu, S. Huang, W. Deng, C.S. Ng, S. Gupta, W. Liu, J.E. Dancey, J.J. Wright, R.L. Bassett, P. Hwu, K.B. Kim, Phase I Study of the Combination of Sorafenib and Temsirolimus in Patients with Metastatic Melanoma. Clin. Cancer Res. 18, 1120–1128 (2012). https://doi.org/10.1158/1078-0432.CCR-11-2436
S.K. Kumar, J. Jett, R. Marks, R. Richardson, F. Quevedo, T. Moynihan, G. Croghan, S.N. Markovic, K.C. Bible, R. Qin, A. Tan, J. Molina, S.H. Kaufmann, C. Erlichman, A.A. Adjei, Phase 1 study of sorafenib in combination with bortezomib in patients with advanced malignancies. Investig. N. Drugs 31, 1201–1206 (2013). https://doi.org/10.1007/s10637-013-0004-2
V. Makker, M.H. Taylor, C. Aghajanian, A. Oaknin, J. Mier, A.L. Cohn, M. Romeo, R. Bratos, M.S. Brose, C. DiSimone, M. Messing, D.E. Stepan, C.E. Dutcus, J. Wu, E.V. Schmidt, R. Orlowski, P. Sachdev, R. Shumaker, A. Casado Herraez, Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer. JCO 38, 2981–2992 (2020). https://doi.org/10.1200/JCO.19.02627
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
This study is a secondary analysis of published data and does not require ethics committee approval.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Acitelli, E., Maiorca, C., Grani, G. et al. Metabolic adverse events of multitarget kinase inhibitors: a systematic review. Endocrine 81, 16–29 (2023). https://doi.org/10.1007/s12020-023-03362-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03362-2