Abstract
Purpose:
Well-differentiated lung neuroendocrine tumors (Lu-NET) are classified as typical (TC) and atypical (AC) carcinoids, based on mitotic counts and necrosis. However, prognostic factors, other than tumor node metastasis (TNM) stage and the histopathological diagnosis, are still lacking. The current study is aimed to identify potential prognostic factors to better stratify lung NET, thus, improving patients’ treatment strategy and follow-up.
Methods:
A multicentric retrospective study, including 300 Lung NET, all surgically removed, from Italian and Spanish Institutions.
Results:
Median age 61 years (13–86), 37.7% were males, 25.0% were AC, 42.0% were located in the lung left parenchyma, 80.3% presented a TNM stage I-II. Mitotic count was ≥2 per 10 high-power field (HPF) in 24.7%, necrosis in 13.0%. Median overall survival (OS) was 46.1 months (0.6–323), median progression-free survival (PFS) was 36.0 months (0.3–323). Female sex correlated with a more indolent disease (T1; N0; lower Ki67; lower mitotic count and the absence of necrosis). Left-sided primary tumors were associated with higher mitotic count and necrosis. At Cox-multivariate regression model, age, left-sided tumors, nodal (N) positive status and the diagnosis of AC resulted independent negative prognostic factors for PFS and OS.
Conclusions:
This study highlights that laterality is an independent prognostic factors in Lu-NETs, with left tumors being less frequent but showing a worse prognosis than right ones. A wider spectrum of clinical and pathological prognostic factors, including TNM stage, age and laterality is suggested. These parameters could help clinicians to personalize the management of Lu-NET.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroendocrine neoplasms of the lung (lung NENs) are classified according to 2021 WHO classification [1] into four major groups, depending on morphology, mitotic count, and necrosis.
Well-differentiated (WD) lung neuroendocrine tumors (Lu-NET) are categorized in typical (TC) and atypical (AC) carcinoids [1]. The differences between these two entities are defined according to mitotic count and the evaluation of occurrence and extent of necrosis (with mitotic count <2 per 10 high-power field (HPF) and absence of necrosis for the diagnosis of TC and mitotic count ≥2 per 10 HPF and/or the presence of necrosis for the diagnosis of AC). Poorly differentiated (PD) tumors are sub-classified into small cells and large cells lung neuroendocrine carcinomas (SCLCs and LCNECs), depending on cellular size. PD lung NEN are characterized by high mitotic count (>10 per 10 HPF), the presence of necrosis and are associated with an aggressive behavior and a dismal prognosis [2].
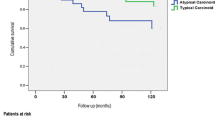
To date, the most relevant prognostic factor for Lu-NET is the histological diagnosis of TC or AC [3]. TC are considered indolent tumors with low recurrence rates and favorable prognosis, whereas AC are a more heterogeneous group in terms of clinical presentation and course, response to therapies and survival [4]. Surgery is the treatment of choice for both TC and AC, with a 5- and 10-year survival rate greater than 90% for TC and a 5-year survival rate ranging from 56 to 87% in AC [4, 5].
Traditionally, other factors have been established for their prognostic value, such as tumor node metastasis (TNM) stage [6] and especially nodal status [7]. However, TNM staging itself has demonstrated to be insufficient to correctly stratify Lu-NET. Thereby, the need to integrate pathological and molecular features to TNM staging has been suggested by several recent works [8, 9].
Many efforts have been made to deepen the molecular landscape of Lu-NETs. In the context of sporadic Lu-NETs, genome sequencing analyses have illustrated that chromatin-remodeling is the foremost as often as possible deregulated pathway, and MEN1 [10], PSIP1 and ARID1A the most commonly mutated genes [11]. Another study has suggested a potentially relevant role for other genes, as BCL2 and BCL2/BAX, associated with anti-apoptotic activity, as independent prognostic parameters in Lu-NETs [12].
Interestingly, some studies have showed how the genetic alterations of TC differ from those of AC [13, 14]. A study conducted with next generation sequencing (NGS) on 148 lung NENs, including 88 TC and AC, demonstrated that MEN1 alterations were almost exclusive to WD Lu-NETs, whereas alterations of TP53 and RB1 genes were significantly enriched in SCLC and LCNECs [15]. However, in this analysis AC showed a hybrid pattern, whereby gains of TERT, SDHA, RICTOR, PIK3CA, MYCL, and SRC were found at rates similar to those in PD lung NENs. More recently, a comprehensive molecular characterization of a large population of Lu-NET has been carried out. In this study, the authors, through machine learning and multi-omics factor analysis, have identified that according to tumor genomic profile a group of AC should be considered similar to PD, high-grade LCNEC [16]. This group has been defined as “supracarcinoids” and has resulted associated with a dismal prognosis.
In the same direction, increasing evidences are arising about an intrinsic heterogeneity within Lu-NET. Beyond the morphological classification, the Ki67 index has emerged as a key feature to stratify Lu-NET [17, 18], as also observed in gastro-entero-pancreatic (GEP) NET [19, 20]. Lu-NET with a Ki67 > 20% have been associated with a significantly worse outcome than those with Ki67 ≤ 20% [21, 22].
Moreover, immunostaining for neuroendocrine markers such as chromogranin A, synaptophysin, or transcriptional thyroid factor 1 (TTF-1) have been identified in pulmonary NETs [23]. Unfortunately, a prognostic value for these tissue biomarkers has not been confirmed [2].
Furthermore, a prognostic role for different clinical factors, as age, sex, primary tumor location (central vs. peripheral, left vs. right lung), or pathological features as tumor grade, Ki67 index or mitotic count itself has not been established, so far [24, 25].
In the current study, we retrospectively analyze the features of 300 Lu-NET, from Italian and Spanish Institutions, to identify potential prognostic factors to better stratify Lu-NET patients and, in this way, improving patients’ treatment strategy and follow-up.
Materials and methods
A multicentric retrospective study, including 300 Lu-NET, all surgically removed, from 8 Italian (San Luigi Gonzaga Hospital, Orbassano; Policlinico Umberto I, “Sapienza” University of Rome; European Neuroendocrine Tumor Society (ENETS) Center of Excellence, Sant’Andrea University Hospital, Rome; ENETS Center of Excellence, University of Naples Federico II, Naples; Regina Elena National Cancer Institute, Rome, University of Ferrara, Ferrara; San Camillo Forlanini Hospital, Rome and Mauriziano Hospital, Turin) and 1 Spanish (University Hospital 12 de Octubre, Madrid) NET-referral Institutions.
Patients population
We retrospectively included all consecutive patients with a histologically confirmed diagnosis of WD Lu-NET (codified as TC or AC, according to 2015 WHO classification) and surgical removal of primary tumor. Extra-pulmonary NET, non-operated NET and PD NEN were excluded.
Based on these inclusion and exclusion criteria, we enrolled 420 patients, diagnosed and treated at one of the selected Italian or Spanish Institutions. After excluding patients with no resected primary tumor, PD neuroendocrine carcinomas and with no-neuroendocrine histology, patients that underwent surgery for the purpose of biopsy, 330 patients were eligible for analysis. Furthermore, after excluding patients with insufficient data, a total of 300 patients diagnosed with Lu-NET constituted the study population. Patient selection schema is summarized in Fig. 1.
Clinical and pathological variables considered
Detailed patients’ characteristics were collected, including age, sex, and smoking habit. Tumoral features, comprehending tumor location central or peripheral, the location of the primary tumor in left or right lung parenchyma, TNM stage, “T” parameter itself (size of the primary tumor), “N” parameter itself (nodal involvement by the tumor), 18-FDG-PET-CT and 68-Gallium-DOTATOC PET-CT positivity, date of diagnosis and date of surgery were gathered. All relevant histopathological features as mitotic count, necrosis, Ki67 index, grading and immunohistochemical (IHC) evaluation of Chromogranin A, Synaptophysin and TTF-1 were collected. Progression-free survival (PFS) and overall survival (OS) were gathered.
Statistical analysis
Categorical variables are expressed as number (percentage). Continuous variables are presented as median (range). PFS and OS were estimated using the Kaplan–Meier method; PFS was calculated from the date of surgery to the date of first progression according to RECIST criteria v1.1, or disease-related death for PFS; and OS to the date of death or last follow-up. We performed Chi square test to identify associations between different variables. A p-value of <0.05 was considered statistically significant. Univariate analyses were performed using Cox regression test for each variable of interest, to detect the impact on PFS and OS. For continuous parameters, the threshold was defined as the median value of the population. Multivariate analyses using a Cox proportional hazards regression analysis were performed to identify factors independently associated with prognosis. The results from the survival analyses are presented with the effect estimates, hazard ratios (HR), and 95% confidence interval [95%CI]. All statistical analyses were performed using IBM-SPSS version 25 (IBM Corporation, New-York, United States of America).
Results
Patients characteristics
Three-hundred patients with surgically removed Lu-NET were included in the study. The median age was 61 years (range from 13–86 years), the sex was male in 113 cases (37.7%). Primary tumor side was left in 126 cases (42.0%); AC were 44 (58.6%) left-sided and 31 right-sided. TNM stage at diagnosis was stage I in 171 cases (57.0%) and 65 were N + (21.7%).
Two-hundred ninety-three patients (97.7%) had a complete surgical removal of the primary tumor. The type of surgical resection was lobectomy in 194 (64.7%) of cases, pneumonectomy in 12 (4.0%), bilobectomy in 13 (4.3%), sleeve resection in 10 (3.3%), segmental resection in 13 (4.3%), wedge resection in 27 (9.0%), and other types of surgical resections in 9 cases (3.0%).
The mitotic count was ≥2 per 10 HPF in 74 cases (24.7%), the necrosis was confirmed in 39 cases (13.0%). Ki67 was 1–2% in 143 (47.7%), 3–19% in 80 (26.7%), 20% in 1 case (0.3%) and >20% in 9 cases (3.0%). The mOS was 46.1 months (0.6–323) and the mPFS was 36.0 months (0.3–323).
Patients’ clinical characteristics are summarized in Table 1. Pathological features and surgical data (intervention yes/no, resection margins and type of surgery) of the study population are detailed in Fig. 2a, b, respectively.
The pathological findings of two illustrative cases are depicted in Fig. 3.
a–c Typical lung carcinoid. Endobronchial, right-sided, lesion (a) showing an organoid architecture (b) and low Ki-67 proliferative index (c). d–f Atypical carcinoid lung carcinoid. Peripheral, left-sided, lesion (d) with well-differentiated morphology (e) and high Ki-67 proliferative index (exceeding 20%) (f) a, d: original magnification x100; b, c, f: original magnification x200; e: original magnification x400
Correlation with key variables: sex and primary tumor side
In Table 2, we report the associations between tumor side and patients’ sex, and the other variables determined by Chi square test.
Sex was associated with smoking habit (p = 0.002), tumor location (p = 0.025), T (p = 0.004), N (p < 0.0001), grade (p < 0.0001), Ki67 (p = 0.002), mitotic count (p = 0.001), and necrosis (p = 0.008). Female sex correlated with a more indolent disease (T1 vs. T2-T3-T4; N0 vs. N positive; G1-2 vs. G3, Ki67 of 1–2%, mitotic count <2 per 10 HPF vs. ≥2 per 10 HPF and the absence of necrosis) and no smoking habit. Conversely, male sex was associated with a more aggressive disease (8/9 patients with Ki67 > 20% were males, as well as 9/10 patients with G3).
Primary tumor side was associated with mitotic count (p = 0.039) and the presence of necrosis (p < 0.0001). Right primary tumor side presented at diagnosis in 60.1% of cases with mitotic count <2 per 10 HPF. Conversely, the presence of necrosis was confirmed in 74.3% of cases in left-sided primary tumors.
The associations with patients’ age, peripheral vs. central location of the primary tumor and necrosis are detailed in the Supplementary materials.
Correlation with type of surgical resection
We also assessed the associations between the type of surgery and the other variables determined by Chi square test. The type of surgical removal was associated with primary tumor location central vs. peripheral (p < 0.001), with lobectomy performed in 130 central lesions (of 193 cases that underwent to lobectomy). All cases (n = 10) treated with sleeve resection were centrally located, whereas 20 of 16 cases who received a wedge resection presented a peripheral location. Type of surgery resulted associated also with T (p < 0.001), N (p = 0.001) and stage at diagnosis (p < 0.001). One-hundred sixty-nine of 186 patients that underwent to lobectomy presented a TNM stage I or II. Lobectomy, segmental, and wedge resection correlated with TC diagnosis (p = 0.008). Finally, lobectomy was associated with a mitotic count <2 per 10 HPF (p = 0.017) and to the absence of necrosis (p = 0.002).
Prognostic impact on PFS and OS
Cox-univariate regression model
We detected a significant impact on patients’ outcome for sex, with male sex associated with dismal PFS (p < 0.0001) and OS (p < 0.0001) and for age, with a worse PFS for patient older than median age value (p = 0.038) and a worse OS for progressively higher age value (considered as continue variable, p = 0.012). A relevant impact for primary tumor side was found, with a negative prognostic impact for left-sided tumors (PFS p = 0.007, OS p = 0.014). Regarding the TNM stage at diagnosis, T2-3-4 vs. T1 (p < 0.0001, p = 0.011), N+ vs. N0 (p < 0.0001, p < 0.0001) and stage II-III-IV vs. stage I (p < 0.0001, p < 0.0001), correlated with shorter PFS and OS, respectively. Mitotic count ≥2 per 10 HPF vs. lower mitotic rate (p < 0.0001, p = 0.002), tumor grading (with G3 vs. G1-2: p < 0.0001, p < 0.0001), a Ki67 > 20% vs. lower Ki67 value (p = 0.001, p < 0.0001), the presence of necrosis vs. its absence (p < 0.0001, p = 0.003) and the diagnosis of AC vs. the diagnosis of TC (p < 0.0001, p < 0.0001), correlated with shorter PFS and OS. The positive immunostaining for Chromogranin A was associated with better OS (p = 0.022). Mitotic count >10 per 10 HPF (p < 0.0001) correlated with shorter PFS. Other variables, such as smoking habit, the type of surgical resection or primary tumor location central vs. peripheral were not significant in terms of patients’ outcomes.
Survival curves in terms of PFS and OS for the variables with significant impact on survival are reported in Fig. 4a (PFS: clinical and pathological variables) and Fig. 4b (OS: clinical and pathological variables). Additionally, we have explored the survival curves for mitotic count >10 per 10 HPF and Ki67 > 20%. These survival curves are depicted in the Supplementary materials.
a Kaplan–Meier survival curves of the clinic-pathological variables with significant impact on patient’s survival in terms of PFS. p-values have been considered as significant if <0.05 and are reported according to the corresponding univariant Cox regression analysis. b Kaplan–Meier survival curves of the clinic-pathological variables with significant impact on patient’s survival in terms of OS. p-values have been considered as significant if <0.05 and are reported according to the corresponding univariant Cox regression analysis
Cox-multivariate regression analysis
At Cox-multivariate regression analysis, a progressively age value (considered as continue variable) p = 0.023, HR: 1.043 (95% CI, 1.006–1.082), tumor side (left vs. right) p = 0.043, HR: 3.033 (95% CI, 1.034–8.893) and N+ vs. N0 p = 0.003, HR: 6.481 HR (95% CI, 1.878–22.374) resulted independent negative prognostic factors for OS.
Additionally, a progressively age value p = 0.027, HR: 1.025 HR (95% CI, 1.003–1.047), tumor side (left vs. right) p = 0.071, HR: 1.823 (95% CI, 0.951–3.494), T 2-3-4 vs. T1 p = 0.000, HR: 2.064 (95% CI, 1.469–2.899) and N+ vs. N0 p = 0.011, HR: 2.598 (95% CI, 1.246.5.420) resulted independent negative prognostic factors in terms of PFS. The diagnosis of TC vs. AC was a protective factor for PFS (p = 0.002, HR: 0.281, 95% CI, 0.125–0.631).
The details of Cox-univariate and -multivariate regression analysis, in terms of OS and PFS, for variables with significant p-value are reported in Table 3(a) (OS) and (b) (PFS).
Discussion
Lu-NETs, account for ~30% of all NETs, 8.8% of all lung NENs (where SCLC accounts for 80% and LCNEC for 11.2%) and about 1–2% of all cancers of pulmonary origin [26]. Lu-NETs, given to their WD morphology and low proliferation index in the majority of cases are associated with an indolent clinical course and a relatively good prognosis if compared with PD lung NEC and also to the other subtypes of no-neuroendocrine lung cancers (as adenocarcinoma and squamous cell carcinoma) [27].
However, despite the existence of established morphological and immunohistochemical criteria for histopathological diagnosis for TC and AC (according to 2021 WHO classification), there is a lack of universal consensus for prognostic factors that could be useful for clinicians to better stratify these patients and to optimize their treatment strategy [28]. Therefore, the identification and the validation of reproducible clinical and pathological prognostic factors represents an urgent unmet need.
TNM stage, T, N status, and tumor morphology (AC vs. TC): a confirmation of validated prognostic factors for Lu-NETs
TNM cancer staging is one of the most relevant and established prognostic tools across several types of tumors. TNM system quantify the extent of the primary tumor (T), lymph nodes (N), and distant metastases (M), providing a stage grouping [29]. TNM stage groups are of crucial importance in oncology [30]. Worsening survival rates with increasing TNM status have been demonstrated in all cancers [31, 32].
In the largest series of NET [33] and NEC [34], that are data obtained from the Surveillance, Epidemiology, and End Results (SEER) program, TNM stage has been confirmed as a fundamental prognostic marker, independently from primary tumor origin.
Given to their low incidence, Lu-NETs do not have a tumor-specific staging system. However, since the 7th edition, the TNM classification for lung cancer has been used for TC and AC, providing a clear stratification of patients with a major impact on patients’ outcomes [35]. In our study, TNM staging has been confirmed as a well-established prognostic factor for survival. This data was consistent with literature data [36].
In this context, a relevant role is represented by nodal status [7]. In a recent retrospective study, including 3.335 Lu-NETs, a positive nodal status emerged as independent negative prognostic factor (HR: 2.3), both for TC and AC [37]. In our study, N+ was associated with worse outcomes with a significant impact on PFS and OS, p = 0.011, HR: 2.598 and p = 0.003, HR: 6.481, respectively.
To date, the diagnosis of TC vs. AC represents the major indicator to guide the clinicians in their management of these patients. TC are associated with a biologically more indolent disease with a significant better prognosis if compared to AC [38,39,40]. A reason could be found in the recurrence rates, accounting for 16.6% in AC and 4.9% in TC [41]. A retrospective analysis of 62 AC, demonstrated that after a complete surgically removal, recurrences were observed mostly within the first 5 years of follow-up, within bronchi, mediastinal nodes, the liver, and bones [42]. In our study, the diagnosis of TC vs. AC clearly depicts two different groups, with a well-defined gap in terms of patients’ outcomes. Notably, the diagnosis of AC was confirmed as an independent negative prognostic factor in terms of PFS.
Sex and age: challenging clinical factors
In our analysis, male sex correlated with a more aggressive disease, with a significant impact on survival. This observation, that clearly is showed in Kaplan–Meier curves of PFS and OS (p < 0.0001 and p < 0.0001, respectively). However, its independent value was not confirmed in our multivariant Cox regression model. According to SEER data, female patients were more likely to have a primary NET located in the lungs respect to males [43]. This data is confirmed in our study, where 62.3% of the patients included were female. Notably, a sex difference between TC and AC in males and females has been reported in literature, with a higher percentage of AC between males [44]. Several works report a correlation between male sex and a worse prognosis for Lu-NETs. This data is coherent with what observed in the no-neuroendocrine counterpart of lung tumors [45]. Filosso et al. reported the male sex as a negative prognostic factor for Lu-NETs [46]. In the same direction, the data coming from a retrospective analysis of 293 Lu-NETs, where OS was influenced by male sex. A recent study confirmed sex as independent factor for OS [47]. In this analysis female patients presented better prognosis than male (HR = 0.604, 95% CI = 0.45–0.806). On the other hand, in the Cox regression univariate analysis of 108 Lu-NET patients, sex was not associated with patient survival [48]. Recent analyses [49] have reported clinically relevant differences between male and female in NET originating from other primary sites (i.e., pancreatic NET).
Additionally, our analysis showed a relevant role for patients’ age. Our multivariate model confirmed the independent value of age, associating a higher risk of progression and death for increasing value of this variable. A role for age as a prognostic factor for NET has been previously reported [50, 51]. Focusing on Lu-NET, a retrospective analysis including TC selected from the SEER database, allowed to develop a nomogram to predict the probability of 3- and 5-year OS [52]. In this model longer OS was associated with younger age (p < 0.0001). This data has been confirmed by other studies, as a retrospective analysis of 108 Lu-NET [53] and an Asiatic study, including 64 lung carcinoids [54].
Therefore, considering our results and the available literature data, we suggest that sex and age should be evaluated as potential useful prognostic factors for Lu-NETs. Perspective larger studies are encouraged to validate this observation.
Old parameters and new openings: mitotic count, necrosis, and Ki67 value
According to 2021 WHO classification, Lu-NETs are divided in two entities (TC and AC) according to two parameters, the mitotic count and the presence/absence of necrosis. These criteria are largely different from the ones for the diagnosis of NET with other origin [55]. For extra-pulmonary (EP) NETs the proliferation activity is quantified trough Ki67 index, which together with morphology, allows the codification of a grade for each case. Therefore, EP-NETs are separated in three classes, with a progressively higher Ki67 value, named G1, G2, and G3 NETs.
For Lu-NETs, several attempts have been made to introduce tumor grading and Ki67 to obtain a uniform classification for all sites NETs [56]. Some data exists about the utility of Ki67 as a prognostic biomarker even for Lu-NETs [57]. However, to date this type of classification has not been validated yet.
In our study, the two traditional pathological features for Lu-NETs, the mitotic count and the necrosis, confirmed their value also as prognostic factors. A mitotic count ≥2 per 10 HPF and the presence of necrosis correlated with a dismal outcome (in terms of PFS p < 0.0001 both, and OS p = 0.002 and p = 0.03).
Notably, in our study, 8 (2.7%) patients had a mitotic count >10 per 10 HPF. This subgroup presented a significant worse prognosis if compared to patients with lower mitotic count. This observation is intriguing, due to the 2021 WHO classification criteria that consider the mitotic count cut-off for Lu-NETs, equal to a maximum of 10 mitosis for 10 HPF. Taking together these data, a more heterogeneous and complex scenario could be hypothesized, comprehending Lu-NET characterized by a WD morphology associated with a high mitotic rate and a poor outcome.
Additionally, in our analysis 9 (3.0%) cases were reported to have a Ki67 > 20%. Among them, 5 presented a mitotic count ≤10 per 10 HPF, 2 a mitotic count ≥10 per 10 HPF (one of 12 and one of 14 per 10 HPF), this data was missing in the remaining 2 cases. This population of patients correlated with dismal outcome, in terms of PFS and OS, p = 0.006 and p < 0.0001, respectively. The Ki67 in this subgroup presented a median value of 30% and a range from 25% to 80%. Therefore, the highest Ki67 value in our study was 80%. This data differs from previous evidences, which reported lower maximum Ki67 index (65% in the study by Rubino et al. [58], 62% in the study by Oka et al. [21], 37% in the study by Kasajima et al. [22]). Arising literature data suggest that Lu-NET with a Ki67 > 20% are associated with a worse clinical outcome, if compared to WD-Lu-NET with lower Ki67 index [19]. An example is the study carried out by Rubino, with mOS of 203 months in patients with Ki67 index ≤5%, 101 months in patients with Ki67 index of 6–20% and 53 months in patients with Ki67 > 20% (p = 0.002) [58].
Therefore, the value of Ki67 as well as the role of mitotic count higher than 10 per 10 HPF, still remains an open and challenging question for Lu-NETs. A more complete diagnosis, taking into account tumor heterogeneity, immunohistochemistry and genomic profile, could help to provide new weapons to better characterize and stratify Lu-NETs, in the era of personalized medicine.
New perspectives: primary tumor side, left-sided vs. right-sided tumors
Laterality is a potential significant prognostic factor for lung cancer because of the heterogeneity between left and right lung, in terms of anatomy, hematic and lymphatic circulation, relationship to the surrounding organs. The importance of right-sided vs. left-sided tumors has been clearly demonstrated for colon cancer, which exhibits differences in incidence, pathogenesis, molecular pathways and outcome depending on the location of the tumor [59]. A prognostic difference between right and left cancer has been also observed for the renal cell carcinoma by analyzing the SEER database. The worse prognosis in left than right tumors was related to earlier tumor stage, lower tumor grade, lymph node and distant metastasis [60].
However, a role for the side of primary tumor in the context of lung cancers in general and specifically for Lu-NET has not been established, so far. Previous studies in the field of Lu-NETs have demonstrated that right-sided tumors are more common [61], in analogy to our population (in which 172 cases were right-sided, accounting for 57.3% of the included population). A recent study analyzed the prognostic relevance of laterality in 1465 patients with non-small cell lung cancer undergone pneumonectomy [62]. Although right-sided pneumonectomy after induction therapy was associated with a significantly higher perioperative mortality, the 5-year survival was similar bewteen tumor sides. Anyway, this previous observation is not significant for the present study, due to the very low rate of pneumonectomy performed in lung NETs.
In our study, a highly significant association between tumor side and the presence of necrosis was found. In 39 of 300 cases, focal or diffuse necrosis was observed. 29 of them (74.3%) were detected in left-sided tumors, whereas only 10 (25.6%) in right-sided primaries. We observed also a significant negative impact for left tumor side on patients’ prognosis at the univariant analysis (PFS, p = 0.007 and OS p = 0.014). Therefore, we confirmed left side as an independent negative prognostic factor in terms of PFS (p = 0.043, HR: 3.033 (95% CI, 1.034–8.893) and OS p = 0.071, HR: 1.823 (95% CI, 0.951–3.494) at the multivariate analysis. The distribution of AC was in 44 in the left parenchyma and in 31 cases in right parenchyma. Among them, 39 of 75 cases were positive for necrosis at the histopathological evaluation, whereas the remaining 36 cases were diagnosed as AC due to the mitotic count ≥2 per 10 HPF only. Thereby, the simple distribution of AC cases between left and right parenchyma is not sufficient to explain the notably higher presence of necrosis in left tumors.
The consistence of this association between necrosis and left Lu-NETs could provide the rationale for a differential expression of angiogenesis and hypoxia according to the primary side of the tumor (right-sided vs. left-sided) with potentially relevant implications for patients’ outcomes. Therefore, we believe that this association should be better evaluated in further original and prospective studies to determine if primary tumor side could represent a new prognostic marker for Lu-NETs. Moreover, a better knowledge of these tumors biology and angiogenesis could pave the way for a more personalized approach in the therapeutic algorithm of these tumors.
The current study presents some limitations. First of all, the retrospective nature of patients’ inclusion, the collection of patients’ data and of the analysis. A second limit could be represented by patients’ sample size, although relatively adequate if we consider that NENs are rare tumors and between them, Lu-NET account for about 25–30%. Another limit is the percentage of missing data above all for pathological variables, as IHC for TTF-1 or Ki67 value (missing in 168 and 68 cases, respectively).
Conclusions
This study highlights that laterality could be included among the prognostic factors with potential clinical relevance in Lu-NETs. The traditionally validated prognostic factors as TNM stage, nodal status and tumor morphology also confirmed their prognostic role. Left lung NETs showed a significantly higher rate of tumor necrosis than right tumors, suggesting a molecular basis for the negative outcomes of the formers. This finding needs to be investigated more in deep to establish the pathogenic rationale of the left-right difference. Other potentially relevant prognostic factors, which need to be further investigated are proliferative index, male sex, and age.
Data availability
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
WHO Classification of Tumors Editorial Board. Thoracic tumours. 5th edn. (International Agency for Research on Cancer World Health Organisation, Lyon, 2021).
J. Wang, L. Ye, H. Cai, M. Jin, Comparative study of large cell neuroendocrine carcinoma and small cell lung carcinoma in high-grade neuroendocrine tumors of the lung: a large population-based study. J. Cancer 10(18), 4226–4236 (2019). https://doi.org/10.7150/jca.33367
P.L. Filosso, O. Rena, G. Donati, C. Casadio, E. Ruffini, E. Papalia et al. Bronchial carcinoid tumors: surgical management and long-term outcome. J. Thorac. Cardiovasc. Surg. 123(2), 303–309 (2002). https://doi.org/10.1067/mtc.2002.119886
M.E. Caplin, E. Baudin, P. Ferolla, P. Filosso, M. Garcia-Yuste, E. Lim et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann. Oncol. Off. J. Eur. SocMed. Oncol. 26(8), 1604–1620 (2015). https://doi.org/10.1093/annonc/mdv041
E.M. Wolin, Challenges in the diagnosis and management of well-differentiated neuroendocrine tumors of the lung (typical and atypical carcinoid): current status and future considerations. The Oncologist 20(10), 1123–1131 (2015). https://doi.org/10.1634/theoncologist.2015-0198
O. Abdel-Rahman, Modified staging system for pulmonary carcinoids on the basis of lung cancer TNM system. Clin. Transl. Oncol. Off. Publ. Fed. Span Oncol. Soc. Natl Cancer Inst. Mex. 20(5), 670–677 (2018). https://doi.org/10.1007/s12094-017-1759-2
P.J. Kneuertz, M.K. Kamel, B.M. Stiles, B.E. Lee, M. Rahouma, S.W. Harrison et al. Incidence and prognostic significance of carcinoid lymph node metastases. Ann. Thorac. Surg. 106(4), 981–988 (2018). https://doi.org/10.1016/j.athoracsur.2018.05.044
M. Cattoni, E. Vallières, L.M. Brown, A.A. Sarkeshik, S. Margaritora, A. Siciliani et al. Improvement in TNM staging of pulmonary neuroendocrine tumors requires histology and regrouping of tumor size. J. Thorac. Cardiovasc. Surg. 155(1), 405–413 (2018). https://doi.org/10.1016/j.jtcvs.2017.08.102
A.S. Jackson, A. Rosenthal, M. Cattoni, A.J. Bograd, A.S. Farivar, R.W. Aye et al. Staging system for neuroendocrine tumors of the lung needs to incorporate histologic grade. Ann. Thorac. Surg. 109(4), 1009–1018 (2020). https://doi.org/10.1016/j.athoracsur.2019.09.053
H. Qiu, B.-M. Jin, Z.-F. Wang, B. Xu, Q.-F. Zheng, L. Zhang et al. MEN1 deficiency leads to neuroendocrine differentiation of lung cancer and disrupts the DNA damage response. Nat. Commun. 11(1), 1009 (2020). https://doi.org/10.1038/s41467-020-14614-4
L. Fernandez-Cuesta, M. Peifer, X. Lu, R. Sun, L. Ozretić, D. Seidal et al. Frequent mutations in chromatin-remodelling genes in pulmonary carcinoids. Nat. Commun. 5, 3518 (2014). https://doi.org/10.1038/ncomms4518
M. Velinovic, R. Jankovic, D. Jovanovic, V. Skodric Trifunovic, D. Gavrilovic, J. Stojsic et al. Tumor characteristics, expressions of ERCC1, Bax, p53, IGF1R, Bcl2, Bcl2/Bax and prognostic factors for overall survival in patients with lung carcinoid. J. BUON 24(1), 256–266 (2019).
A.K. Walch, H.F. Zitzelsberger, M.M. Aubele, A.E. Mattis, M. Bauchinger, S. Candidus et al. Typical and atypical carcinoid tumors of the lung are characterized by 11q deletions as detected by comparative genomic hybridization. Am. J. Pathol. 153(4), 1089–1098 (1998). https://doi.org/10.1016/S0002-9440(10)65653-2
D. Moris, I. Ntanasis-Stathopoulos, D.I. Tsilimigras, M.A. Adam, C.-F.J. Yang, D. Harpole et al. Insights into novel prognostic and possible predictive biomarkers of lung neuroendocrine tumors. Cancer Genom. Proteom. 15(2), 153–163 (2018). https://doi.org/10.21873/cgp.20073
M. Simbolo, A. Mafficini, K.O. Sikora, M. Fassan, S. Barbi, V. Corbo et al. Lung neuroendocrine tumours: deep sequencing of the four World Health Organization histotypes reveals chromatin-remodelling genes as major players and a prognostic role for TERT, RB1, MEN1 and KMT2D. J. Pathol. 241(4), 488–500 (2017). https://doi.org/10.1002/path.4853
N. Alcala, N. Leblay, A.A.G. Gabriel, L. Mangiante, D. Hervas, T. Giffon et al. Integrative and comparative genomic analyses identify clinically relevant pulmonary carcinoid groups and unveil the supra-carcinoids. Nat. Commun. 10(1), 3407 (2019). https://doi.org/10.1038/s41467-019-11276-9
J.Y. Kim, S.-M. Hong, J.Y. Ro, Recent updates on grading and classification of neuroendocrine tumors. Ann. Diagn. Pathol. 29, 11–16 (2017). https://doi.org/10.1016/j.anndiagpath.2017.04.005
M. Volante, G. Gatti, M. Papotti, Classification of lung neuroendocrine tumors: lights and shadows. Endocrine 50(2), 315–319 (2015). https://doi.org/10.1007/s12020-015-0578-x
B.C.M. Hermans, J.L. Derks, L. Moonen, C.H.J. Habraken, J. der Thüsen, L.M. von, Hillen et al. Pulmonary neuroendocrine neoplasms with well differentiated morphology and high proliferative activity: illustrated by a case series and review of the literature. Lung Cancer Amst. Neth. 150, 152–158 (2020). https://doi.org/10.1016/j.lungcan.2020.10.015
T. Feola, R. Centello, F. Sesti, G. Puliani, M. Verrico, V. Di Vito et al. Neuroendocrine carcinomas with atypical proliferation index and clinical behavior: a systematic review. Cancers 13(6), 1247 (2021). https://doi.org/10.3390/cancers13061247
N. Oka, A. Kasajima, B. Konukiewitz, A. Sakurada, Y. Okada, T. Kameya et al. Classification and prognostic stratification of bronchopulmonary neuroendocrine neoplasms. Neuroendocrinology 110(5), 393–403 (2020). https://doi.org/10.1159/000502776
A. Kasajima, B. Konukiewitz, N. Oka, H. Suzuki, A. Sakurada, Y. Okada et al. Clinicopathological profiling of lung carcinoids with a Ki67 Index > 20. Neuroendocrinology 108(2), 109–120 (2019). https://doi.org/10.1159/000495806
M.T. Siddiqui, Pulmonary neuroendocrine neoplasms: a review of clinicopathologic and cytologic features. Diagn. Cytopathol. 38(8), 607–617 (2010). https://doi.org/10.1002/dc.21244
G. Pelosi, A. Fabbri, M. Cossa, A. Sonzogni, B. Valeri, L. Righi et al. What clinicians are asking pathologists when dealing with lung neuroendocrine neoplasms? Semin. Diagn. Pathol. 32(6), 469–479 (2015). https://doi.org/10.1053/j.semdp.2015.10.009
L. Righi, M. Volante, I. Rapa, S. Vatrano, G. Pelosi, M. Papotti, Therapeutic biomarkers in lung neuroendocrine neoplasia. Endocr. Pathol. 25(4), 371–377 (2014). https://doi.org/10.1007/s12022-014-9335-6
A.E. Hendifar, A.M. Marchevsky, R. Tuli, Neuroendocrine tumors of the lung: current challenges and advances in the diagnosis and management of well-differentiated disease. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 12(3), 425–436 (2017). https://doi.org/10.1016/j.jtho.2016.11.2222
S. Singh, E.K. Bergsland, C.M. Card, T.A. Hope, P.L. Kunz, D.T. Laidley et al. Commonwealth Neuroendocrine Tumour Research Collaboration and the North American Neuroendocrine Tumor Society Guidelines for the Diagnosis and Management of Patients With Lung Neuroendocrine Tumors: An International Collaborative Endorsement and Update of the 2015 European Neuroendocrine Tumor Society Expert Consensus Guidelines. J Thorac Oncol Off Publ Int Assoc Study. Lung Cancer 15(10), 1577–1598 (2020). https://doi.org/10.1016/j.jtho.2020.06.021
E. Baudin, A.R. Hayes, J.-Y. Scoazec, P.L. Filosso, E. Lim, G. Kaltsas et al. Unmet medical needs in pulmonary neuroendocrine (carcinoid) neoplasms. Neuroendocrinology. 108(1), 7–17 (2019). https://doi.org/10.1159/000493980
S.B. Edge, C.C. Compton, The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 17(6), 1471–1474 (2010). https://doi.org/10.1245/s10434-010-0985-4
M. Piñeros, D.M. Parkin, K. Ward, E. Chokunonga, M. Ervik, H. Farrugia et al. Essential TNM: a registry tool to reduce gaps in cancer staging information. Lancet Oncol. 20(2), e103–e111 (2019). https://doi.org/10.1016/S1470-2045(18)30897-0
R. Rami-Porta, H. Asamura, W.D. Travis, V.W. Rusch, Lung cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 67(2), 138–155 (2017). https://doi.org/10.3322/caac.21390
G.N. Hortobagyi, S.B. Edge, A. Giuliano, New and important changes in the TNM staging system for breast cancer. Am. Soc. Clin. Oncol. Educ. Book Am. Soc. Clin. Oncol. Annu. Meet. 38, 457–467 (2018). https://doi.org/10.1200/EDBK_201313
A. Dasari, C. Shen, D. Halperin, B. Zhao, S. Zhou, Y. Xu et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 3(10), 1335 (2017). https://doi.org/10.1001/jamaoncol.2017.0589
A. Dasari, K. Mehta, L.A. Byers, H. Sorbye, J.C. Yao, Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: a SEER database analysis of 162,983 cases. Cancer 124(4), 807–815 (2018). https://doi.org/10.1002/cncr.31124
J.Y. Yoon, K. Sigel, J. Martin, R. Jordan, M.B. Beasley, C. Smith et al. Evaluation of the prognostic significance of tnm staging guidelines in lung carcinoid tumors. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 14(2), 184–192 (2019). https://doi.org/10.1016/j.jtho.2018.10.166
J.K. Dermawan, C.F. Farver, The prognostic significance of the 8th edition TNM staging of pulmonary carcinoid tumors: a single institution study with long-term follow-up. Am. J. Surg. Pathol. 43(9), 1291–1296 (2019). https://doi.org/10.1097/PAS.0000000000001268
C.F. Thomas, H.D. Tazelaar, J.R. Jett, Typical and atypical pulmonary carcinoids: outcome in patients presenting with regional lymph node involvement. Chest 119(4), 1143–1150 (2001). https://doi.org/10.1378/chest.119.4.1143
H. Asamura, T. Kameya, Y. Matsuno, M. Noguchi, H. Tada, Y. Ishikawa et al. Neuroendocrine neoplasms of the lung: a prognostic spectrum. J. Clin. Oncol. 24(1), 70–76 (2006). https://doi.org/10.1200/JCO.2005.04.1202
G. Fink, T. Krelbaum, A. Yellin, D. Bendayan, M. Saute, M. Glazer et al. Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest 119(6), 1647–1651 (2001). https://doi.org/10.1378/chest.119.6.1647
W.D. Travis, W. Rush, D.B. Flieder, R. Falk, M.V. Fleming, A.A. Gal et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am. J. Surg. Pathol. 22(8), 934–944 (1998). https://doi.org/10.1097/00000478-199808000-00003
F. Davini, A. Gonfiotti, C. Comin, A. Caldarella, F. Mannini, A. Janni, Typical and atypical carcinoid tumours: 20-year experience with 89 patients. J. Cardiovasc. Surg. (Torino) 50(6), 807–811 (2009).
F. Marciello, O. Mercier, P. Ferolla, J.-Y. Scoazec, P.L. Filosso, A. Chapelier et al. Natural history of localized and locally advanced atypical lung carcinoids after complete resection: a joined French-Italian Retrospective Multicenter Study. Neuroendocrinology 106(3), 264–273 (2018). https://doi.org/10.1159/000480015
J.C. Yao, M. Hassan, A. Phan, C. Dagohoy, C. Leary, J.E. Mares et al. One hundred years after ‘carcinoid’: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 26(18), 3063–3072 (2008). https://doi.org/10.1200/JCO.2007.15.4377
C. Kesrouani, C. Ghorra, M. Rassy, H.R. Kourie, J. Kattan, Distribution and characteristics of pulmonary neuroendocrine tumors: single institution experience in Lebanon. Asian Pac. J. Cancer Prev. APJCP 17(5), 2579–2581 (2016)
N. Pendleton, M.F. Jefferson, G.R. Dixon, M.W. Myskow, M.A. Horan, Correlates of tumor size, sex, cell type, and metastasis of resected non-small cell lung cancer and age. J. Gerontol. A Biol. Sci. Med. Sci. 51(1), B50–B53 (1996). https://doi.org/10.1093/gerona/51a.1.b50
P.L. Filosso, E. Ruffini, S. Di Gangi, F. Guerrera, G. Bora, G. Ciccone et al. Prognostic factors in neuroendocrine tumours of the lung: a single-centre experience. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 45(3), 521–526 (2014). https://doi.org/10.1093/ejcts/ezt442
M. Chiappetta, I. Sperduti, L.P. Ciavarella, G. Leuzzi, E. Bria, F. Mucilli et al. Prognostic score for survival with pulmonary carcinoids: the importance of associating clinical with pathological characteristics. Interact. Cardiovasc. Thorac. Surg. 31(3), 315–323 (2020). https://doi.org/10.1093/icvts/ivaa114
V.E. Georgakopoulou, E. Zygouris, C. Nikokiris, C. Damaskos, A. Pierrakou, N. Garmpis et al. Predictive indicators of survival in patients with surgically resected lung carcinoid tumors at a Greek Medical Center. Cureus 12(9), e10300 (2020). https://doi.org/10.7759/cureus.10300
G. Muscogiuri, B. Altieri, M. Albertelli, A. Dotto, R. Modica, L. Barrea et al. Epidemiology of pancreatic neuroendocrine neoplasms: a sex perspective. Endocrine 69(2), 441–450 (2020). https://doi.org/10.1007/s12020-020-02331-3
Z. Yang, W. Wang, J. Lu, G. Pan, Z. Pan, Q. Chen et al. Gastric neuroendocrine tumors (G-Nets): incidence, prognosis and recent trend toward improved survival. Cell Physiol. Biochem. Int. J. Exp. Cell Physiol. Biochem. Pharmacol. 45(1), 389–396 (2018). https://doi.org/10.1159/000486915
D. Valvi, X. Mei, M. Gupta, M.B. Shah, A. Ancheta, F. Marti et al. Younger age is associated with improved survival in patients undergoing liver transplantation alone for metastatic neuroendocrine tumors. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 25(6), 1487–1493 (2021). https://doi.org/10.1007/s11605-020-04708-1
L. Cao, Z.-W. Li, M. Wang, T.-T. Zhang, B. Bao, Y.-P. Liu, Clinicopathological characteristics, treatment and survival of pulmonary large cell neuroendocrine carcinoma: a SEER population-based study. PeerJ. 7, e6539 (2019)
M. Peri, E. Botteri, E. Pisa, F. De Marinis, A. Ungaro, F. Spada et al. A single-institution retrospective analysis of metachronous and synchronous metastatic bronchial neuroendocrine tumors. J. Thorac. Dis. 10(7), 3928–3939 (2018). https://doi.org/10.21037/jtd.2018.06.78
B.-S. Wu, Y. Hu, J. Sun, J.-L. Wang, P. Wang, W.-W. Dong et al. Analysis on the characteristics and prognosis of pulmonary neuroendocrine tumors. Asian Pac. J. Cancer Prev. APJCP 15(5), 2205–2210 (2014). https://doi.org/10.7314/apjcp.2014.15.5.2205
G. Rindi, D.S. Klimstra, B. Abedi-Ardekani, S.L. Asa, F.T. Bosman, E. Brambilla et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 31(12), 1770–1786 (2018). https://doi.org/10.1038/s41379-018-0110-y
S. La Rosa, S. Uccella, Classification of neuroendocrine neoplasms: lights and shadows. Rev. Endocr. Metab. Disord. 22(3), 527–538 (2021). https://doi.org/10.1007/s11154-020-09612-2
O. Sazonova, V. Manem, M. Orain, B. Khoshkrood-Mansoori, N. Gaudreault, P. Desmeules et al. Transcriptomic data helps refining classification of pulmonary carcinoid tumors with increased mitotic counts. Mod. Pathol. 33(9), 1712–1721 (2020). https://doi.org/10.1038/s41379-020-0538-8
M. Rubino, J.Y. Scoazec, E. Pisa, M. Faron, L. Spaggiari, J. Hadoux et al. Lung carcinoids with high proliferative activity: further support for the identification of a new tumor category in the classification of lung neuroendocrine neoplasms. Lung Cancer Amst. Neth. 148, 149–158 (2020). https://doi.org/10.1016/j.lungcan.2020.08.001
S.Y. Yang, M.S. Cho, N.K. Kim, Difference between right-sided and left-sided colorectal cancers: from embryology to molecular subtype. Expert Rev. Anticancer. Ther. 18(4), 351–358 (2018). https://doi.org/10.1080/14737140.2018.1442217
S. Guo, K. Yao, X. He, S. Wu, Y. Ye, J. Chen, C.L. Wu, Prognostic significance of laterality in renal cell carcinoma: A population-based study from the surveillance, epidemiology, and end results (SEER) database. Cancer Med. 8(12), 5629–5637 (2019). https://doi.org/10.1002/cam4.2484
B.I. Gustafsson, M. Kidd, A. Chan, M.V. Malfertheiner, I.M. Modlin, Bronchopulmonary neuroendocrine tumors. Cancer 113(1), 5–21 (2008). https://doi.org/10.1002/cncr.23542
C.J. Yang, S.A. Shah, B.K. Lin, K.W. VanDusen, D.Y. Chan, W.D. Tan, D.N. Ranney, T.A. Cox ML,D’Amico, M.F. Berry, Right-sided versus left-sided pneumonectomy after induction therapy for non-small cell lung cancer. Ann. Thorac. Surg. 107(4), 1074–1081 (2019). https://doi.org/10.1016/j.athoracsur.2018.10.009
Author information
Authors and Affiliations
Contributions
All authors contributed to the development of the publication and maintained control over the final content. The authors did not receive professional help with the preparation of the manuscript. Individual contributions to the paper (according to CRediT roles): A.L.S., I.P., A.S., M.V., M.B., R.M., A.A., I.Z., B.T.M., E.T., G.P., M.R., E.P., R.B., T.F., F.S., P.R., R.M., M.M., F.P., M.V., E.G., C.R., M.A., A.I., F.V., M.R.A., M.C.Z., M.I., A.C., M.P.B., R.G.C., A.F. Conceptualization: A.L.S., M.P.B., A.F. Data curation: A.L.S., I.P., A.S., M.V., M.B., R.M., A.A., I.Z., B.T.M., E.T., G.P., M.R., E.P., R.B., T.F., F.S., P.R., R.M., M.M. Formal analysis: A.L.S., C.R., A.F. Methodology: A.L.S., A.F. Project administration: F.P., E.G., A.F. Software: A.L.S., A.F. Supervision: M.V., A.F. Validation: M.V., E.G., M.A., A.I., F.V., M.R.A., M.C.Z., M.I., A.C., M.P.B., R.G.C., A.F. Visualization: A.L.S., A.F. Roles/writing—original draft: A.L.S., A.F. Writing—review & editing: A.L.S., I.P., A.S., M.V., M.B., R.M., A.A., I.Z., B.T.M., E.T., G.P., M.R., E.P., R.B., T.F., F.S., P.R., R.M., M.M., F.P., M.V., E.G., C.R., M.A., A.I., F.V., M.R.A., M.C.Z., M.I., A.C., M.P.B., R.G.C., A.F.
Funding
This study was partially supported by the Italian ministerial research project PRIN2017Z3N3YC. Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Institutional review board statement
This study complies with the guidelines for human studies and it was conducted ethically in accordance with the Declaration of Helsinki and approved by the Federico II University Ethics Committee (protocol no. 227/2011).
Informed consent
All patients gave written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
La Salvia, A., Persano, I., Siciliani, A. et al. Prognostic significance of laterality in lung neuroendocrine tumors. Endocrine 76, 733–746 (2022). https://doi.org/10.1007/s12020-022-03015-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03015-w