Abstract
Purpose
The role of ATP, which is secreted by pancreatic β-cells, is still a matter of debate. It has been postulated that extracellular ATP acts as a positive auto- or paracrine signal in β-cells amplifying insulin secretion. However, there is rising evidence that extracellular ATP may also mediate a negative signal.
Methods
We evaluated whether extracellular ATP interferes with the Ca2+-mediated negative feedback mechanism that regulates oscillatory activity of β-cells.
Results
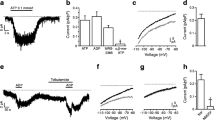
To experimentally uncover the Ca2+-induced feedback we applied a high extracellular Ca2+ concentration. Under this condition ATP (100 µM) inhibited glucose-evoked oscillations of electrical activity and hyperpolarized the membrane potential. Furthermore, ATP acutely increased the interburst phase of Ca2+ oscillations and reduced the current through L-type Ca2+ channels. Accordingly, ATP (500 µM) decreased glucose-induced insulin secretion. The ATP effect was not mimicked by AMP, ADP, or adenosine. The use of specific agonists and antagonists and mice deficient of large conductance Ca2+-dependent K+ channels revealed that P2X, but not P2Y receptors, and Ca2+-dependent K+ channels are involved in the underlying signaling cascade induced by ATP. The effectiveness of ATP to interfere with parameters of stimulus-secretion coupling is markedly reduced at low extracellular Ca2+ concentration.
Conclusion
It is suggested that extracellular ATP which is co-secreted with insulin in a pulsatile manner during glucose-stimulated exocytosis provides a negative feedback signal driving β-cell oscillations in co-operation with Ca2+ and other signals.









Similar content being viewed by others
Abbreviations
- V m :
-
cell membrane potential
- Δψ :
-
mitochondrial membrane potential
- [Ca2+]c :
-
cytosolic Ca2+ concentration
- BK-KO:
-
BK channel knock-out
References
K. Sawada, N. Echigo, N. Juge, T. Miyaji, M. Otsuka, H. Omote, A. Yamamoto, Y. Moriyama, Identification of a vesicular nucleotide transporter. Proc. Natl. Acad. Sci. USA 105(15), 5683–5686 (2008). https://doi.org/10.1073/pnas.0800141105
J.C. Hutton, E.J. Penn, M. Peshavaria, Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem. J. 210(2), 297–305 (1983)
J.W. Leitner, K.E. Sussman, A.E. Vatter, F.H. Schneider, Adenine nucleotides in the secretory granule fraction of rat islets. Endocrinology 96(3), 662–677 (1975). https://doi.org/10.1210/endo-96-3-662
S. Obermuller, A. Lindqvist, J. Karanauskaite, J. Galvanovskis, P. Rorsman, S. Barg, Selective nucleotide-release from dense-core granules in insulin-secreting cells. J. Cell. Sci. 118(Pt 18), 4271–4282 (2005). https://doi.org/10.1242/jcs.02549
M. Cieslak, K. Roszek, Purinergic signaling in the pancreas and the therapeutic potential of ecto-nucleotidases in diabetes. Acta Biochim. Pol. 61(4), 655–662 (2014)
J.R. Weitz, M. Makhmutova, J. Almaca, J. Stertmann, K. Aamodt, M. Brissova, S. Speier, R. Rodriguez-Diaz, A. Caicedo, Mouse pancreatic islet macrophages use locally released ATP to monitor beta cell activity. Diabetologia 61(1), 182–192 (2018). https://doi.org/10.1007/s00125-017-4416-y
P. Petit, A.D. Lajoix, R. Gross, P2 purinergic signalling in the pancreatic beta-cell: control of insulin secretion and pharmacology. Eur. J. Pharm. Sci. 37(2), 67–75 (2009). https://doi.org/10.1016/j.ejps.2009.01.007
P. Petit, G. Bertrand, W. Schmeer, J.C. Henquin, Effects of extracellular adenine nucleotides on the electrical, ionic and secretory events in mouse pancreatic beta-cells. Br. J. Pharmacol. 98(3), 875–882 (1989)
C.R. Poulsen, K. Bokvist, H.L. Olsen, M. Hoy, K. Capito, P. Gilon, J. Gromada, Multiple sites of purinergic control of insulin secretion in mouse pancreatic beta-cells. Diabetes 48(11), 2171–2181 (1999)
S. Amisten, S. Meidute-Abaraviciene, C. Tan, B. Olde, I. Lundquist, A. Salehi, D. Erlinge, ADP mediates inhibition of insulin secretion by activation of P2Y13 receptors in mice. Diabetologia 53(9), 1927–1934 (2010). https://doi.org/10.1007/s00125-010-1807-8
M. Ohtani, K. Ohura, T. Oka, Involvement of P2X receptors in the regulation of insulin secretion, proliferation and survival in mouse pancreatic beta-cells. Cell. Physiol. Biochem. 28(2), 355–366 (2011). https://doi.org/10.1159/000331752
M. Ohtani, J. Suzuki, K.A. Jacobson, T. Oka, Evidence for the possible involvement of the P2Y(6) receptor in Ca (2+) mobilization and insulin secretion in mouse pancreatic islets. Purinergic Signal 4(4), 365–375 (2008). https://doi.org/10.1007/s11302-008-9122-2
C. Leon, M. Freund, O. Latchoumanin, A. Farret, P. Petit, J.P. Cazenave, C. Gachet, The P2Y(1) receptor is involved in the maintenance of glucose homeostasis and in insulin secretion in mice. Purinergic Signal 1(2), 145–151 (2005). https://doi.org/10.1007/s11302-005-6209-x
J. Meister, D. Le Duc, A. Ricken, R. Burkhardt, J. Thiery, H. Pfannkuche, T. Polte, J. Grosse, T. Schoneberg, A. Schulz, The G protein-coupled receptor P2Y14 influences insulin release and smooth muscle function in mice. J. Biol. Chem. 289(34), 23353–23366 (2014). https://doi.org/10.1074/jbc.M114.580803
F. Blachier, W.J. Malaisse, Effect of exogenous ATP upon inositol phosphate production, cationic fluxes and insulin release in pancreatic islet cells. Biochim. Biophys. Acta 970(2), 222–229 (1988)
P. Petit, D. Hillaire-Buys, M. Manteghetti, S. Debrus, J. Chapal, M.M. Loubatieres-Mariani, Evidence for two different types of P2 receptors stimulating insulin secretion from pancreatic B cell. Br. J. Pharmacol. 125(6), 1368–1374 (1998). https://doi.org/10.1038/sj.bjp.0702214
P. Petit, M. Manteghetti, R. Puech, M.M. Loubatieres-Mariani, ATP and phosphate-modified adenine nucleotide analogues. Effects on insulin secretion and calcium uptake. Biochem. Pharmacol. 36(3), 377–380 (1987)
Y.F. Zhao, R. Xu, M. Hernandez, Y. Zhu, C. Chen, Distinct intracellular Ca2+ response to extracellular adenosine triphosphate in pancreatic beta-cells in rats and mice. Endocrine 22(3), 185–192 (2003). https://doi.org/10.1385/ENDO:22:3:185
E. Grapengiesser, H. Dansk, B. Hellman, Pulses of external ATP aid to the synchronization of pancreatic beta-cells by generating premature Ca(2+) oscillations. Biochem. Pharmacol. 68(4), 667–674 (2004). https://doi.org/10.1016/j.bcp.2004.04.018
B. Hellman, H. Dansk, E. Grapengiesser, Pancreatic beta-cells communicate via intermittent release of ATP. Am. J. Physiol. Endocrinol. Metab. 286(5), E759–765 (2004). https://doi.org/10.1152/ajpendo.00452.2003
E. Gylfe, E. Grapengiesser, H. Dansk, B. Hellman, The neurotransmitter ATP triggers Ca2 + responses promoting coordination of pancreatic islet oscillations. Pancreas 41(2), 258–263 (2012). https://doi.org/10.1097/MPA.0b013e3182240586
B. Gier, P. Krippeit-Drews, T. Sheiko, L. Aguilar-Bryan, J. Bryan, M. Düfer, G. Drews, Suppression of KATP channel activity protects murine pancreatic beta-cells against oxidative stress. J. Clin. Invest. 119(11), 3246–3256 (2009). https://doi.org/10.1172/JCI38817
A. Edalat, P. Schulte-Mecklenbeck, C. Bauer, S. Undank, P. Krippeit-Drews, G. Drews, M. Düfer, Mitochondrial succinate dehydrogenase is involved in stimulus-secretion coupling and endogenous ROS formation in murine beta cells. Diabetologia 58(7), 1532–1541 (2015). https://doi.org/10.1007/s00125-015-3577-9
G. Grynkiewicz, M. Poenie, R.Y. Tsien, A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260(6), 3440–3450 (1985)
M. Düfer, D. Haspel, P. Krippeit-Drews, L. Aguilar-Bryan, J. Bryan, G. Drews, Oscillations of membrane potential and cytosolic Ca2+ concentration in SUR1-/- beta cells. Diabetologia 47(3), 488–498 (2004). https://doi.org/10.1007/s00125-004-1348-0
J.C. Henquin, Glucose-induced electrical activity in beta-cells. Feedback control of ATP-sensitive K+ channels by Ca2+? [corrected]. Diabetes 39(11), 1457–1460 (1990)
P. Krippeit-Drews, M. Düfer, G. Drews, Parallel oscillations of intracellular calcium activity and mitochondrial membrane potential in mouse pancreatic B-cells. Biochem. Biophys. Res. Commun. 267(1), 179–183 (2000). doi:10.1006/bbrc.1999.1921 S0006-291X(99)91921-6 [pii]
T.D. Plant, Properties and calcium-dependent inactivation of calcium currents in cultured mouse pancreatic B-cells. J. Physiol. 404, 731–747 (1988)
Maczewsky, J., Sikimic, J., Bauer, C., Krippeit-Drews, P., Wolke, C., Lendeckel, U., Barthlen, W., Drews, G. The LXR ligand T0901317 acutely inhibits insulin secretion by affecting mitochondrial metabolism. Endocrinology (2017). https://doi.org/10.1210/en.2016-1941
A. Salehi, S.S. Qader, E. Grapengiesser, B. Hellman, Inhibition of purinoceptors amplifies glucose-stimulated insulin release with removal of its pulsatility. Diabetes 54(7), 2126–2131 (2005)
M. Düfer, B. Gier, D. Wolpers, P. Krippeit-Drews, P. Ruth, G. Drews, Enhanced glucose tolerance by SK4 channel inhibition in pancreatic beta-cells. Diabetes 58, 1835–1843 (2009). doi:db08-1324 [pii] 10.2337/db08-1324
M. Düfer, Y. Neye, K. Hörth, P. Krippeit-Drews, A. Hennige, H. Widmer, H. McClafferty, M.J. Shipston, H.U. Häring, P. Ruth, G. Drews, BK channels affect glucose homeostasis and cell viability of murine pancreatic beta cells. Diabetologia 54(2), 423–432 (2011). https://doi.org/10.1007/s00125-010-1936-0
G. Burnstock, Purinergic signalling in endocrine organs. Purinergic Signal 10(1), 189–231 (2014). https://doi.org/10.1007/s11302-013-9396-x
G. Burnstock, Purine and pyrimidine receptors. Cell. Mol. Life Sci. 64(12), 1471–1483 (2007). https://doi.org/10.1007/s00018-007-6497-0
S.N. Yang, P.O. Berggren, The role of voltage-gated calcium channels in pancreatic beta-cell physiology and pathophysiology. Endocr. Rev. 27(6), 621–676 (2006). https://doi.org/10.1210/er.2005-0888
E. Gylfe, B. Hellman, External ATP mimics carbachol in initiating calcium mobilization from pancreatic beta-cells conditioned by previous exposure to glucose. Br. J. Pharmacol. 92(2), 281–289 (1987)
N. Porksen, M. Hollingdal, C. Juhl, P. Butler, J.D. Veldhuis, O. Schmitz, Pulsatile insulin secretion: detection, regulation, and role in diabetes. Diabetes 51(Suppl 1), S245–254 (2002)
A. Hazama, S. Hayashi, Y. Okada, Cell surface measurements of ATP release from single pancreatic beta cells using a novel biosensor technique. Pflug. Arch. 437(1), 31–35 (1998)
K. Nakazawa, K. Fujimori, A. Takanaka, K. Inoue, An ATP-activated conductance in pheochromocytoma cells and its suppression by extracellular calcium. J. Physiol. 428, 257–272 (1990)
J.F. Rolland, J.C. Henquin, P. Gilon, Feedback control of the ATP-sensitive K+ current by cytosolic Ca2+ contributes to oscillations of the membrane potential in pancreatic beta-cells. Diabetes 51(2), 376–384 (2002)
Q. Gong, M. Kakei, N. Koriyama, M. Nakazaki, S. Morimitsu, K. Yaekura, C. Tei, P2Y-purinoceptor mediated inhibition of L-type Ca2+ channels in rat pancreatic beta-cells. Cell Struct. Funct. 25(5), 279–289 (2000)
M.C. Jacques-Silva, M. Correa-Medina, O. Cabrera, R. Rodriguez-Diaz, N. Makeeva, A. Fachado, J. Diez, D.M. Berman, N.S. Kenyon, C. Ricordi, A. Pileggi, R.D. Molano, P.O. Berggren, A. Caicedo, ATP-gated P2X3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic beta cell. Proc. Natl. Acad. Sci. USA 107(14), 6465–6470 (2010). doi:0908935107 [pii] 10.1073/pnas.0908935107
J. Fernandez-Alvarez, D. Hillaire-Buys, M.M. Loubatieres-Mariani, R. Gomis, P. Petit, P2 receptor agonists stimulate insulin release from human pancreatic islets. Pancreas 22(1), 69–71 (2001)
Khan, S., Yan-Do, R., Duong, E., Wu, X., Bautista, A., Cheley, S., MacDonald, P.E., Braun, M. Autocrine activation of P2Y receptors couples Ca influx to Ca release in human pancreatic beta cells. Diabetologia (2014). https://doi.org/10.1007/s00125-014-3368-8
P.E. MacDonald, M. Braun, J. Galvanovskis, P. Rorsman, Release of small transmitters through kiss-and-run fusion pores in rat pancreatic beta cells. Cell. Metab. 4(4), 283–290 (2006). https://doi.org/10.1016/j.cmet.2006.08.011
Acknowledgements
We are grateful to Isolde Breuning for excellent technical assistance. BK-KO mice were kindly provided by Prof. Dr. Peter Ruth, Institute of Pharmacy, University of Tübingen.
Author's contribution
C.B., J.K., and J.S. researched data; P.K.-D. evaluated data and edited the manuscript; M.D. contributed to discussion and study design and edited the manuscript; G.D. designed the study, wrote and edited the manuscript, and contributed to discussion. G.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Bauer, C., Kaiser, J., Sikimic, J. et al. ATP mediates a negative autocrine signal on stimulus-secretion coupling in mouse pancreatic β-cells. Endocrine 63, 270–283 (2019). https://doi.org/10.1007/s12020-018-1731-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1731-0