Abstract
Pancreatic β cells express several P2 receptors including P2Y1 and the modulation of insulin secretion by extracellular nucleotides has suggested that these receptors may contribute to the regulation of glucose homeostasis. To determine whether the P2Y1 receptor is involved in this process, we performed studies in P2Y1 mice. In baseline conditions, P2Y1-mice exhibited a 15% increase in glycemia and a 40% increase in insulinemia, associated with a 10% increase in body weight, pointing to a role of the P2Y1 receptor in the control of glucose metabolism. Dynamic experiments further showed that P2Y1-mice exhibited a tendency to glucose intolerance. These features were associated with a decrease in the plasma levels of free fatty acid and triglycerides. When fed a lipids and sucrose enriched diet for 15 weeks, the two genotypes no longer displayed any significant differences. To determine whether the P2Y1 receptor was directly involved in the control of insulin secretion, experiments were carried out in isolated Langerhans islets. In the presence of high concentrations of glucose, insulin secretion was significantly greater in islets from P2Y1-mice. Altogether, these results show that the P2Y1 receptor plays a physiological role in the maintenance of glucose homeostasis at least in part by regulating insulin secretion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extracellular nucleotides regulate biological processes as diverse as smooth muscle contraction, neurotransmission, the immune response, inflammation, platelet aggregation, pain or the modulation of cardiac functions by acting on specific receptors [1]. These so-called P2 receptors are divided into two families: The metabotropic P2Y and ionotropic P2X receptors. P2Y receptors are 7-transmembrane domain, G protein-coupled polypeptides, of which eight subtypes have been cloned and characterized to date, differing in their sequence, pharmacological properties and intracellular signaling. The first P2Y receptor to be cloned [2], thus named P2Y1, is an ADP receptor expressed in a wide range of tissues which activates PLCβ through Gq coupling [3, 4]. Although the precise physiological function of this receptor in most tissues is not yet known, its role in hemostasis and thrombosis is now firmly established [5]. The P2Y1 receptor is expressed in platelets [3] and together with the P2Y12 receptor plays an important role in the platelet aggregation induced by ADP or other aggregating agents [6–9]. Thus, P2Y1-deficient mice and wild-type (wt) mice treated with a P2Y1 antagonist are resistant to thrombosis in several experimental models [9–13].
A growing body of evidence suggests that P2 receptors may be involved in the regulation of glucose homeostasis. Pancreatic β cells express a wide range of P2 receptors including P2Y subtypes [14], while stable analogues of ATP and ADP have been shown to increase insulin secretion and decrease glycemia in vivo [15]. In addition, potent P2Y1 agonists have been described as insulin secretagogues in isolated rat pancreas [16], suggesting the functional participation of a P2Y1-like receptor in this mechanism. A role of P2Y receptors in glucose transport has also been proposed in rat cardiomyocytes [17] and human skin fibroblasts [18].
To determine whether the P2Y1 receptor subtype is involved in the regulation of glucose homeostasis, we performed studies in P2Y1-/- mice. P2Y1 deficiency was found to result in increases in glycemia and insulinemia in the nonfasting state, associated with increased body weight and decreased plasma levels of free fatty acids and triglycerides. In vitro experiments in isolated pancreatic islets showed an increased insulin secretion in P2Y1-deficient mice, suggesting a role of the P2Y1 receptor in the maintenance of glucose homeostasis and in the regulation of insulin secretion.
Materials and methods
Animals
Wild-type and P2Y1-deficient male and female mice were raised as described previously [9] and had a C57BL/6 background at the F7 generation. Animals used in the experiments were aged-matched (10–12 weeks old except otherwise stated), housed at 22 °C under a 12-h light/dark cycle and had free access to food and water. The mice were normally fed a standard laboratory chow containing about 3% fat (Safe, Epinay/Orge, France). Body weight was monitored by weighing the mice from weaning (21 days) to 12 months and food intake by weighing the grid metal food container every 2–3 days for 2 weeks and dividing the result by the number of mice per cage (three mice) and the number of days. The high fat/high sucrose diet (HF/HS), which was administered for 15 weeks, contained 24% fat (hydrogenated coconut oil) and was enriched in sucrose (30%) (Research Diets, New Brunswick, USA).
Lipid measurements
After an 8-h fast, mice were anesthetized with methoxyflurane and blood was drawn from the abdominal aorta into heparin lithium (20 U/ml) as anticoagulant. Plasma triglycerides, total cholesterol and HDL-cholesterol were determined using an Olympus AU400 analyzer (Olympus SA, Rungis, France) with kits and controls supplied by Olympus. Free fatty acids were measured on the AU400 analyzer using a kit from Wako (Richmond, USA).
Plasma glucose
Glucose tolerance was determined following a 24-h fast by i.p. administration of glucose (3 g/kg) and measurement of plasma glucose at 15, 30, 60 and 120 min. Insulin sensitivity was determined in mice fasted for 4 h by i.p. injection of insulin (Humulin, 0.75 mg/kg; Eli Lilly, France) and measurement of plasma glucose at 15, 30, 60 and 90 min. Blood samples were collected from the tail tip of conscious mice and glucose concentrations were measured using glucose analyzer strips (Accu-Chek active, Roche, Mannheim, Germany).
Insulin measurements
Mice were fasted for 4 h and injected with glucose (3 g/kg). Blood was taken from the orbital plexus of conscious mice at 0, 30 and 60 min and insulin was measured with a monoclonal insulin RIA (Linco Research, St Louis, MO). For the determination of insulin content in the whole pancreas, each mouse pancreas was homogenized in 4 ml of ice-cold acid-ethanol solution (0.15 N HCl:75% ethanol) [19], and insulin was extracted at 4 °C overnight. Insulin concentrations were assayed in 1,000-fold or 10,000-fold diluted samples.
Insulin secretion in isolated pancreatic islets
Langerhans islets from wild-type and P2Y1-deficient female mice were isolated by collagenase digestion of the pancreas according to a technique derived from that of Lacy and Kostianowsky [20]. The islets were stabilized for 45 min at 37 °C in a Krebs-bicarbonate buffer containing (mmol/l) NaCl 108, NaHCO3 18, KCl 4.7, CaCl2 2.5, MgSO4 1.2 and KH2PO4 1.2 (pH 7.4), bubbled with a mixture of O2:CO2 (95%:5%) and supplemented with glucose (5 mmol/l) and bovine serum albumin (Fraction V, 1 mg/ml). Thereafter, batches of three islets from wildtype and P2Y1-/- mice were incubated for 60 min in 1 ml of Krebs-bicarbonate buffer containing different concentrations of glucose (3, 7, 11 and 20 mmol/l). At the end of the incubation period, a sample of the medium was taken for immunoassay of insulin.
Histological examination of mouse pancreas and determination of the percentage of fat mass
For histological examination, mouse pancreas were fixed in 10% buffered neutral formalin and embedded in paraffin. For each pancreas, one slide containing three histological sections was stained with hematoxylin/eosin. The determination of body fat percentage was performed at the Mouse Clinical Institute (Illkirch, France), using a Piximus apparatus (GE Medical System, Madison, Wisconsin, USA).
Statistical analyses
Values are reported as the mean ± SEM and nonparametric Mann-Whitney tests were performed to compare two groups of independent values, and the areas under the GTT curves were calculated to compare the difference between wt and P2Y1-/- mice in the glucose tolerance test. A Wilcoxon matched pairs test was used to compare the difference in body weight between wild-type and P2Y1-/- mice.
Results
Increased glycemia and body weight despite normal food consumption in P2Y1-/- mice
The effect of P2Y1 deficiency on glycemia was assessed according to the nutritional status of the animals. On a normal chow diet, the plasma glucose level in nonfasted animals was significantly increased in P2Y1-/- male mice as compared to wild-type (wt) male mice (9.79 ± 0.33 and 7.95 ± 0.30 mmol/l, respectively, P < 0.01). A similar tendency was observed in P2Y1-/- and wt female mice, although it did not reach statistical significance (8.98 ± 0.23 and 8.15 ± 0.38 mmol/l, respectively) (Figures 1a and b). After a 6-h fast, the difference in glucose level between the two genotypes was still significant in P2Y1-/- and wt males (9.5 ± 0.59 and 7.85 ± 0.29 mmol/l, respectively, P < 0.05), but not in P2Y1-/- and wt females (9.10 ± 0.20 and 7.53 ± 0.48 mmol/l, respectively) (Figures 1a and b). However, after an overnight fast, no significant difference was observed between P2Y1-/- and wt males (4.89 ± 0.19 and 4.56 ± 0.15 mmol/l, respectively) or females (4.92 ± 0.25 and 4.60 ± 0.20 mmol/l, respectively) (not shown). The increase in glycemia in fed and 6-h-fasted mice was not due to an increase in food consumption, which was similar in wt and P2Y1-/- mice (Figure 2a). It was nevertheless associated with a significant 10% increase in body weight for P2Y1-/- as compared to wt male mice, which was maintained for up to 10 months (P < 0.0001) (Figure 2b), whereas no difference was observed between wt and P2Y1-/- females (data not shown). Body fat percentages showed a slight increase for the male P2Y1-/- mice, although the difference did not reach statistical significance (10.7 ± 0.6 and 11.5 ± 0.5% of fat tissue for wt and P2Y1-/-, respectively, n = 4). Similarly, the difference in plasma leptin levels, which reflect the amount of fat tissue, was not statistically different between the two genotypes (Table 1).
Biochemical parameters
A metabolic study revealed that plasma free fatty acids and triglycerides were about 20% lower (P < 0.05 and 0.01, respectively) in P2Y1-/- as compared to wt fasted male mice (Table 1), whereas no significant differences were observed in the nonfasted state (data not shown). No significant difference was observed in fasted or nonfasted female mice, whatever the parameter (Table 1). In view of these results, further dynamic experiments were performed in male mice only.
Glucose tolerance and insulin sensitivity
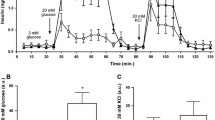
An i.p. glucose challenge (3 g/kg) was administered to determine the dynamic response of P2Y1-/- mice to increased glucose concentrations. A slight glucose intolerance was observed in P2Y1-deficient mice as compared to wt mice (P < 0.05) (Figure 3a). The insulin sensitivity of mice fasted for 4 h was investigated by i.p. injection of insulin (0.75 U/kg) and measurement of plasma glucose concentrations over time. Although P2Y1-/- mice fasted for 4 h still exhibited increased glycemia, the glucose lowering effect of insulin was similar in these animals as compared to wt mice (Figure 3b). Insulin measurements revealed a 40% increased level in P2Y1-/- mice in the nonfasting state (4 h food withdrawal). However, after injection of glucose (3 g/kg), no significant difference in insulin levels was observed between the two genotypes at 30 and 60 min (Table 2).
Glucose tolerance and insulin sensitivity of wt and P2Y1-/- mice. Plasma glucose levels were measured over time following intraperitoneal injection of glucose (3 g/kg) (a, n = 9; P < 0.05) or insulin (0.75 U/kg) (b, n = 7). Values are the means ± SEM. P indicates significant difference between the two area under the curve.
Glucose tolerance, insulin sensitivity and plasma lipids in mice fed an HF/HS diet
To determine whether these slight differences could be exacerbated by diet, wt and P2Y1-deficient mice were fed a high fat/high sucrose diet for 15 weeks. This diet resulted in a significant increase in body weight of wt mice (P < 0.01). However, no more difference in body weight was observed between wt and P2Y1-/- mice fed the enriched diet (Figure 4a). The diet elicited glucose intolerance in both genotypes relative to the respective mice fed a normal chow diet, though after an overnight fast, glycemia remained identical whatever the genotype or diet (Figures 3a and 4b). In addition, no more significant difference in glucose tolerance or insulin sensitivity was observed between wt and P2Y1-/- mice fed the HF/HS diet (Figures 4b and c). After the 4-h fast, glycemia was nevertheless still higher in P2Y1-/- mice fed the enriched diet (Figure 4c). Concerning plasma lipids, neither triglycerides nor free fatty acids displayed any significant difference after the HF/HS diet (Table 1).
Growth curve, glucose tolerance and insulin sensitivity of wt and P2Y1-/- mice fed an HF/HS diet for 15 weeks. (a) The difference in body weight was again significant when comparing wt vs P2Y1-/- mice fed the normal chow diet (n = 9, P < 0.01), or wt mice fed the normal chow diet vs wt mice fed the HF/HS enriched diet (n = 9, P < 0.01), but not when comparing wt vs P2Y1-/- fed the enriched diet. (b, c) Plasma glucose levels were determined after intraperitoneal administration of glucose (3 g/kg) (b) or insulin (0.75 U/kg) (c). Results are the mean ± SEM (n = 9).
Pancreas morphology and insulin content, and insulin secretion in isolated pancreatic islets
Histological analysis of mouse pancreas revealed no difference between wt and P2Y1-/- mice (n = 4) regarding islets morphology. Pancreas weight was similar between the two genotypes (307.6 ± 30.1 and 296.2 ± 27.9 mg of tissue for the wt and P2Y1-/- mouse pancreas, respectively, n = 5). The insulin content of P2Y1-/- mice pancreas was not significantly different from that of wt mice (90.68 ± 7.75 and 142.3 ± 23.32 mg insulin/g tissue for the wt and P2Y1-/- pancreas, respectively, n = 5, P > 0.05). To assess the potential physiological role of the P2Y1 receptor in the function of pancreatic β cells, we compared the insulin responses of pancreatic islets from wt and P2Y1-deficient mice to increasing concentrations of glucose in vitro. The insulin response of wt islets increased with the level of glucose in the medium, from 0.63 ± 0.07 ng/islet per hour at 3 mmol/l glucose to 1.41 ± 0.14 ng/islet per hour at 20 mmol/l glucose (Figure 5). In the presence of a non-stimulating glucose concentration (3 mmol/l), the level of insulin secretion was similar in islets from P2Y1-/- (0.58 ± 0.09 ng/islet per hour) and wt mice. However, in islets from P2Y1-/- mice, the insulin response to a stimulatory glucose concentration (20 mmol/l) was significantly greater (2.0 ± 0.2 ng/islet per hour, P < 0.02) than the secretion in wt islets (Figure 5).
Discussion
In this study, we investigated the effects of P2Y1 deficiency on the regulation of glucose homeostasis in mice. The most striking observations were increased glycemia and insulinemia in non-fasted P2Y1-deficient animals as compared to wt mice, although a difference could no longer be detected after an overnight fast. These metabolic features were associated in males with an increase in body weight for P2Y1-deficient as compared to wt mice, whereas no difference in food consumption was observed between the two genotypes. The occurrence of a significant rise in glycemia together with a 40% increase in insulinemia in P2Y1-deficient mice would favor the presence of a state of insulin resistance induced by deletion of the P2Y1 gene, in the absence of any increase in food intake.
It is worthy of note that the increase in insulin level was evident under basal conditions (4 h post-prandial), but not after an overnight fast or even under dynamic stimulated conditions. In the glucose tolerance test, a slight decrease in tolerance could be observed in the basal state although it should be noted that the test was performed with a relatively high dose of glucose (3 g/kg) which may have emphasized a mild phenotype. In addition, no significant difference in insulin sensitivity was observed. These findings may be explained by a mild defect of glucose transport as suggested by the work of Solini and coworkers [18]; this defect may be functionally relevant only in the post-prandial period. Regarding the increased insulinemia, our in vitro data in isolated islets strongly suggest that pancreatic β-cell function is improved in P2Y1-deficient mice, particularly in the presence of high glucose concentrations, pointing to a putative inhibitory effect of the P2Y1 receptor on insulin secretion. Although it is difficult to directly relate the in vitro data to the in vivo observations, it should be noted that insulinemia of the P2Y1-/- mice did not increase in vivo during a glucose challenge. One explanation for these data is that these results may be partly related to an abnormality in hypothalamic functions, where the P2Y1 receptor was shown to be expressed [21, 22]. The function of islet β cells is indeed controlled by a glucose sensor acting in synergy with signals that integrate messages originating from other tissues, particularly the hypothalamus [23].
The role of extracellular nucleotides in the modulation of pancreatic insulin secretion is well known [24–26]. It has previously been demonstrated that adenine nucleotides amplify insulin secretion by acting on P2 receptors of pancreatic β cells. These receptors were found to belong to the P2Y family, with ADPbS, ATPαS and 2MeSATP being potent agonists in pancreatic islets isolated from rat, dog or man [15, 27–29]. The insulin response triggered by activation of P2Y receptors is also known to depend on the stimulating glucose concentration [29, 30]. Although the subtype of receptor involved and the stimulus-secretion coupling of β-cell P2 receptors have not yet been unequivocally established, potent P2Y1 agonists have been shown to be strong insulin secretagogues in rat pancreas [16]. All these data disagree with our observation that absence of the P2Y1 receptor contributes to increase insulin secretion instead of decreasing it. However, the effects of nucleotides on pancreatic cells appear to differ in the mouse from those observed in other species such as dog, rat or man. In mice, unlike in other species, the extracellular nucleotides ATP and ADP have been shown to inhibit insulin release from islet cells [31]. Similar observations were reported more recently [32], and the authors showed that nucleotides had an inhibitory action on insulin secretion through direct interference with the secretory machinery by activation of the protein phosphatase calcineurin. These results are in accordance with our findings in P2Y1-/- mice, suggesting an autocrine inhibitory role of this receptor in mouse pancreas in the fed state or under glucose stimulated conditions.
P2Y receptor stimulation was recently shown to participate in glucose uptake in human fibroblasts by mediating glucose transporter (GLUT1) translocation and activation [18]. In fibroblasts from type 2 diabetic patients, this P2-mediated glucose transport was defective due to constitutive P2Y desensitization in these cells, while co-incubation with the nucleotide degrading enzyme apyrase restored ATP-stimulated glucose uptake. Such a phenomenon could contribute to maintain hyperglycemia in these patients [18]. Although the P2Y subtype involved was not clearly identified, it would be interesting to determine whether the P2Y1 receptor might participate in glucose transport, which could also contribute to the phenotype observed in P2Y1-/- mice. Desensitization of the P2Y1 receptor is also observed in mice defective for the ectonucleotidase NTPDase1/CD39. These mice were recently reported to exhibit glucose intolerance, insulin resistance and a decrease in 2-deoxyglucose uptake in cultured hepatocytes, which adds evidence for an important role of extracellular nucleotides in glucose homeostasis, probably at least in part through the P2Y1 receptor [33].
Another metabolic feature found in P2Y1-deficient mice was the decrease in plasma free fatty acids (FFA) and triglycerides. Since the demonstration by [34] of the existence of the glucoseYfatty acid cycle and more recently the elucidation of its mechanism [35], the inhibition of glucose metabolism by FFA has been well documented. FFA should not be involved in the insulin resistance of P2Y1-/- mice. On the contrary, the low levels of FFA and triglycerides are consistent with no real insulin resistance as these are normally elevated in insulinresistant states. Concerning the mechanism of the decrease in plasma FFA, a direct effect of P2Y1 receptor depletion in adipose tissue or in the liver cannot be excluded but would appear to be unlikely. Thus, if A1 adenosine receptors have been shown to be expressed in adipocytes and to play an important physiological role in lipolysis control and insulin sensitivity [36], no information is available on the existence of P2Y1 receptors on adipocytes. In the same way, P2Y1 receptors are expressed in hepatocytes where they have recently been shown to stimulate glycogenolysis through activation of glycogen phosphorylase [37] but there is currently no evidence for the role of P2Y1 receptors in fat metabolism. The decrease in plasma FFA in P2Y1-deficient mice may be related to the higher plasma levels of insulin, which is a well-known antilipolytic and lipogenic hormone. This is supported by and could account for the increase in body weight in P2Y1- deficient mice as compared to wt animals. Whether P2Y1-/- mice could have an enhanced utilization or storage of fatty acids remains to be investigated.
In conclusion, our data in the fed and post-prandial state in mice demonstrate a role of the P2Y1 receptor in the maintenance of glucose homeostasis, at least in part through negative regulation of insulin secretion. This receptor may thus function as an autocrine loop for the feedback control of insulin secretion through release of ADP stored in insulin granules, thereby allowing the b cells to rapidly adapt their response to variations in plasma glucose. Nevertheless, we cannot exclude the possibility that the P2Y1 receptor also acts through other metabolic pathways.
References
Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 1998; 50(3): 413-2.
Webb TE, Simon J, Krishek BJ et al. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett 1993; 324(2): 219-5.
Leon C, Hechler B, Vial C et al. The P2Y1 receptor is an ADP receptor antagonized by ATP and expressed in platelets and megakaryoblastic cells. FEBS Lett 1997; 403(1): 26–30.
Hechler B, Vigne P, Leon C et al. ATP derivatives are antagonists of the P2Y1 receptor: Similarities to the platelet ADP receptor. Mol Pharmacol 1998; 53(4): 727-3.
Gachet C. Identification, characterization, and inhibition of the platelet ADP receptors. Int J Hematol 2001; 74(4): 375-1.
Hollopeter G, Jantzen HM, Vincent D et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 2001; 409(6817): 202–7.
Hechler B, Leon C, Vial C et al. The P2Y1 receptor is necessary for adenosine 5-diphosphate-induced platelet aggregation. Blood 1998; 92(1): 152–9.
Jin J, Kunapuli SP. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc Natl Acad Sci USA 1998; 95(14): 8070-.
Leon C, Hechler B, Freund M et al. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y(1) receptor-null mice. J Clin Invest 1999; 104(12): 1731–7.
Fabre JE, Nguyen M, Latour A et al. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nat Med 1999; 5(10): 1199-02.
Lenain N, Freund M, Leon C et al. Inhibition of localized thrombosis in P2Y1-deficient mice and rodents treated with MRS2179, a P2Y1 receptor antagonist. J Thromb Haemost 2003; 1(6): 1144-.
Leon C, Freund M, Ravanat C et al. Key role of the P2Y(1) receptor in tissue factor-induced thrombin-dependent acute thromboembolism: Studies in P2Y(1)-knockout mice and mice treated with a P2Y(1) antagonist. Circulation 2001; 103(5): 718-3.
Leon C, Ravanat C, Freund M et al. Differential involvement of the P2Y1 and P2Y12 receptors in platelet procoagulant activity. Arterioscler Thromb Vasc Biol 2003; 23(10): 1941-.
Coutinho-Silva R, Parsons M, Robson T, Burnstock G. Changes in expression of P2 receptors in rat and mouse pancreas during development and ageing. Cell Tissue Res 2001; 306(3): 373-3.
Petit P, Hillaire-Buys D, Loubatières-Mariani MM, Chapal J. Purinergic receptors and the pharmacology of type 2 diabetes. In Abbrachio M, Williams M (eds): Handbook of Experimental Phamacology: Purinergic and Pyrimidinergic Signalling. Berlin: Springer 2001; 377-1.
Fischer B, Chulkin A, Boyer JL et al. 2-thioether 5-O-(1-thiotriphosphate)adenosine derivatives as new insulin secretagogues acting through P2Y-Receptors. J Med Chem 1999; 42(18): 3636-6.
Fischer Y, Becker C, Loken C. Purinergic inhibition of glucose transport in cardiomyocytes. J Biol Chem 1999; 274(2): 755-1.
Solini A, Chiozzi P, Morelli A et al. Defective P2Y purinergic receptor function: A possible novel mechanism for impaired glucose transport. J Cell Physiol 2003; 197(3): 435-4.
Yamaoka T, Yano M, Yamada T et al. Diabetes and pancreatic tumours in transgenic mice expressing Pa x 6. Diabetologia 2000; 43(3): 332-.
Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 1967; 16(1): 35-.
Kittner H, Franke H, Fischer W et al. Stimulation of P2Y1 receptors causes anxiolytic-like effects in the rat elevated plus-maze: Implications for the involvement of P2Y1 receptor-mediated nitric oxide production. Neuropsychopharmacology 2003; 28(3): 435-4.
Moore DJ, Chambers JK, Wahlin JP et al. Expression pattern of human P2Y receptor subtypes: A quantitative reverse transcription-polymerase chain reaction study. Biochim Biophys Acta 2001; 1521(1-): 107–19.
Penicaud L, Leloup C, Lorsignol A et al. Brain glucose sensing mechanism and glucose homeostasis. Curr Opin Clin Nutr Metab Care 2002; 5(5): 539-3.
Levine RA, Oyama S, Kagan A, Glick SM. Stimulation of insulin and growth hormone secretion by adenine nucleotides in primates. J Lab Clin Med 1970; 75(1): 30-.
Loubatieres-Mariani MM, Chapal J, Lignon F, Valette G. Structural specificity of nucleotides for insulin secretory action from the isolated perfused rat pancreas. Eur J Pharmacol 1979; 59(3-): 277-6.
Chapal J, Loubatieres-Mariani MM. Effects of phosphate-modified adenine nucleotide analogues on insulin secretion from perfused rat pancreas. Br J Pharmacol 1981; 73(1): 105-0.
Bertrand G, Chapal J, Loubatieres-Mariani MM, Roye M. Evidence for two different P2-purinoceptors on beta cell and pancreatic vascular bed. Br J Pharmacol 1987; 91(4): 783-.
Ribes G, Bertrand G, Petit P, Loubatieres-Mariani MM. Effects of 2-methylthio ATP on insulin secretion in the dog in vivo. Eur J Pharmacol 1988; 155(1-): 171-.
Petit P, Hillaire-Buys D, Manteghetti M et al. Evidence for two different types of P2 receptors stimulating insulin secretion from pancreatic B cell. Br J Pharmacol 1998; 125(6): 1368-4.
Fernandez-Alvarez J, Hillaire-Buys D, Loubatieres-Mariani MM et al. P2 receptor agonists stimulate insulin release from human pancreatic islets. Pancreas 2001; 22(1): 69–71.
Petit P, Bertrand G, Schmeer W, Henquin JC. Effects of extracellular adenine nucleotides on the electrical, ionic and secretory events in mouse pancreatic beta-cells. Br J Pharmacol 1989; 98(3): 875-2.
Poulsen CR, Bokvist K, Olsen HL et al. Multiple sites of purinergic control of insulin secretion in mouse pancreatic beta-cells. Diabetes 1999; 48(11): 2171-1.
Thukral C, Kotani K, Csizmadia E et al. Glucose homeostasis in cd39-/- null mice: Role of extracellular nucleotides in mediation of insulin resistance. In 4th International Symposium of Nucleosides and Nucleotides; 2004. Chapel Hill, North Carolina, USA 2004.
Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963; 1:785-.
Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest 2000; 106(2): 171-.
Dong Q, Ginsberg HN, Erlanger BF. Overexpression of the A1 adenosine receptor in adipose tissue protects mice from obesity-related insulin resistance. Diabetes Obes Metab 2001; 3(5): 360-.
Dixon J, Hall JF, Webb TE, Boarder M. Regulation of rat hepatocyte function by P2Y receptors: Focus on control of glycogen phosphorylase and cyclic AMP by 2-MeSADP. J Pharmacol Exp Ther 2004.
Acknowledgement
This work was supported by INSERM and ARMESA. The authors would like to thank Dr René Gross, Miche`le Manteghetti, Catherine Schwartz and Josiane Weber for their contribution to this study and Juliette Mulvihill for reviewing the text of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Léon, C., Freund, M., Latchoumanin, O. et al. The P2Y1 receptor is involved in the maintenance of glucose homeostasis and in insulin secretion in mice. Purinergic Signalling 1, 145–151 (2005). https://doi.org/10.1007/s11302-005-6209-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-005-6209-x