Abstract
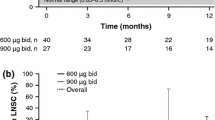
Pasireotide is a multireceptor-targeted somatostatin analog effective in the treatment of Cushing’s disease (CD). We evaluate the value of an acute pasireotide suppression test (PST) in predicting response to medium/long-term treatment in CD. Nineteen patients with active CD were prospectively investigated at two referral centers from May 2013 to August 2014. Follow-up data (median 6 months; range 1–9 months) were available for sixteen patients. All patients received at 09:00 h a single subcutaneous (sc) injection of 600 μg pasireotide. Serum cortisol and plasma ACTH were assessed before, and every 2 h for 8 h after, drug administration. Late-night salivary cortisol (LNSC) was assessed before and after pasireotide administration. After acute PST, all patients were continued on pasireotide 600 μg sc twice a day. During PST, cortisol and ACTH levels quickly decreased in all patients except one with a mean percentage fall, respectively, of 48.9 ± 24.3 and 48.1 ± 25.4 % compared to baseline. LNSC decreased in about 82 % of patients (14/17) achieving a normalization in five of them. Pasireotide treatment was associated with a normalization of 24-h urinary-free cortisol at last follow-up in about 68 % of patients. A fall >27 % of LNSC during PST calculated by ROC curve was the best parameter in predicting a positive response to treatment with pasireotide (sensitivity 91 %; specificity 100 %; positive predictive value 100 %; negative predictive value 75 %). Acute PST may be useful to identify CD patients who will benefit from pasireotide treatment. A LNSC fall >27 % as well as a LNSC normalization during PST is associated with a probability of 100 % of achieving a favorable response to pasireotide treatment in the medium/long term.


Similar content being viewed by others
References
G. Arnaldi, T. Mancini, G. Tirabassi, L. Trementino, M. Boscaro, Advances in the epidemiology, pathogenesis, and management of Cushing’s syndrome complications. J. Endocrinol. Invest. 35(4), 434–448 (2012)
B.M. Biller, A.B. Grossman, P.M. Stewart, S. Melmed, X. Bertagna, J. Bertherat, M. Buchfelder, A. Colao, A.R. Hermus, L.J. Hofland, A. Klibanski, A. Lacroix, J.R. Lindsay, J. Newell-Price, L.K. Nieman, S. Petersenn, N. Sonino, G.K. Stalla, B. Swearingen, M.L. Vance, J.A. Wass, M. Boscaro, Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J. Clin. Endocrinol. Metab. 93(7), 2454–2462 (2008)
A. Colao, M. Boscaro, D. Ferone, F.F. Casanueva, Managing Cushing’s disease: the state of the art. Endocrine 47(1), 9–20 (2014)
A. Colao, S. Petersenn, J. Newell-Price, J.W. Findling, F. Gu, M. Maldonado, U. Schoenherr, D. Mills, L.R. Salgado, B.M. Biller, Pasireotide B2305 Study Group, A 12-month phase 3 study of pasireotide in Cushing’s disease. N. Engl. J. Med. 366(10), 914–924 (2012)
R. Pivonello, S. Petersenn, J. Newell-Price, J.W. Findling, F. Gu, M. Maldonado, A. Trovato, G. Hughes, L.R. Salgado, A. Lacroix, J. Schopohl, B.M. Biller, Pasireotide B2305 Study Group, Pasireotide treatment significantly improves clinical signs and symptoms in patients with Cushing’s disease: results from a Phase III study. Clin. Endocrinol (Oxf) 81(3), 408–417 (2014)
G.F.F.M. Pieters, A.G.H. Smals, P.W.C. Kloppenborg, Long-term treatment of acromegaly with the somatostatin analogue SMS 201–995. N. Engl. J. Med. 314, 1390–1392 (1986)
S.W. Lamberts, P. Uitterlinden, P.C. Schuijff, J.G. Klijn, Therapy of acromegaly with sandostatin: the predictive value of an acute test, the value of serum somatomedin-C measurements in dose adjustment and the definition of a biochemical ‘cure’. Clin. Endocrinol (Oxf) 29, 411–420 (1988)
A. Colao, D. Ferone, S. Lastoria, P. Marzullo, G. Cerbone, A. Di Sarno, S. Longobardi, B. Merola, M. Salvatore, G. Lombardi, Prediction of efficacy of octreotide therapy in patients with acromegaly. J. Clin. Endocrinol. Metab. 81, 2356–2362 (1996)
N. Karavitaki, I. Botusan, S. Radian, M. Coculescu, H.E. Turner, J.A. Wass, The value of an acute octreotide suppression test in predicting long-term responses to depot somatostatin analogues in patients with active acromegaly. Clin. Endocrinol (Oxf) 62, 282–288 (2005)
W.W. de Herder, H.R. Taal, P. Uitterlinden, R.A. Feelders, J.A. Janssen, A.J. van der Lely, Limited predictive value of an acute test with subcutaneous octreotide for long-term IGF-I normalization with Sandostatin LAR in acromegaly. Eur. J. Endocrinol. 153(1), 67–71 (2005)
G. Arnaldi, A. Angeli, A.B. Atkinson, X. Bertagna, F. Cavagnini, G.P. Chrousos, G.A. Fava, J.W. Findling, R.C. Gaillard, A.B. Grossman, B. Kola, A. Lacroix, T. Mancini, F. Mantero, J. Newell-Price, L.K. Nieman, N. Sonino, M.L. Vance, A. Giustina, M. Boscaro, Diagnosis and complications of Cushing’s syndrome: a consensus statement. J. Clin. Endocrinol. Metab. 88(12), 5593–5602 (2003)
L.K. Nieman, B.M. Biller, J.W. Findling, J. Newell-Price, M.O. Savage, P.M. Stewart, V.M. Montori, The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 93(5), 152–640 (2008)
H. Raff, Cushing’s syndrome: diagnosis and surveillance using salivary cortisol. Pituitary 15(1), 64–70 (2012)
J.W. Findling, H. Raff, Cushing’s syndrome: important issues in diagnosis and management. J. Clin. Endocrinol. Metab. 91(10), 3746–3753 (2006)
H. Raff, Update on late-night salivary cortisol for the diagnosis of Cushing’s syndrome: methodological considerations. Endocrine 44(2), 346–349 (2013)
S.A. Doi, J. Clark, A.W. Russell, Concordance of the late night salivary cortisol in patients with Cushing’s syndrome and elevated urine-free cortisol. Endocrine 43(2), 327–333 (2013)
C.A. Carrasco, J. Coste, L. Guignat, L. Groussin, M.A. Dugué, S. Gaillard, X. Bertagna, J. Bertherat, Midnight salivary cortisol determination for assessing the outcome of transsphenoidal surgery in Cushing’s disease. J. Clin. Endocrinol. Metab. 93(12), 4728–4734 (2008)
M.L. Nunes, S. Vattaut, J.B. Corcuff, A. Rault, H. Loiseau, B. Gatta, N. Valli, L. Letenneur, A. Tabarin, Late-night salivary cortisol for diagnosis of overt and subclinical Cushing’s syndrome in hospitalized and ambulatory patients. J. Clin. Endocrinol. Metab. 94(2), 456–462 (2009)
F. Ceccato, N. Albiger, G. Reimondo, A.C. Frigo, S. Ferasin, G. Occhi, F. Mantero, M. Terzolo, C. Scaroni, Assessment of glucocorticoid therapy with salivary cortisol in secondary adrenal insufficiency. Eur. J. Endocrinol. 167(6), 769–776 (2012)
L. Trementino, M. Cardinaletti, C. Concettoni, G. Marcelli, B. Polenta, M. Spinello, M. Boscaro, G. Arnaldi, Salivary cortisol is a useful tool to assess the early response to pasireotide in patients with Cushing’s disease. Pituitary (2014). doi:10.1007/s11102-014-0557-x
R. van der Pas, C. de Bruin, A.M. Pereira, J.A. Romijn, R.T. Netea-Maier, A.R. Hermus, P.M. Zelissen, F.H. de Jong, A.J. van der Lely, W.W. de Herder, S.M. Webb, S.W. Lamberts, L.J. Hofland, R.A. Feelders, Cortisol diurnal rhythm and quality of life after successful medical treatment of Cushing’s disease. Pituitary 16(4), 536–544 (2013)
M. Barbot, N. Albiger, F. Ceccato, M. Zilio, A.C. Frigo, L. Denaro, F. Mantero, C. Scaroni, Combination therapy for Cushing’s disease: effectiveness of two schedules of treatment. Should we start with cabergoline or ketoconazole? Pituitary 17(2), 109–117 (2014)
J. Mackenzie Feder, I. Bourdeau, S. Vallette, H. Beauregard, L.G. Ste-Marie, A. Lacroix, Pasireotide monotherapy in Cushing’s disease: a single-centre experience with 5-year extension of phase III Trial. Pituitary 17, 519–529 (2013)
M. Shenouda, M. Maldonado, Y. Wang, E. Bouillaud, M. Hudson, D. Nesheiwat, K. Hu, An open-label, dose-escalation study of once-daily and twice-daily pasireotide in healthy volunteers: safety, tolerability and effects on glucose, insulin and glucagon levels. Am. J. Ther. 21(3), 164–173 (2014)
R.R. Henry, T.P. Ciaraldi, D. Armstrong, P. Burke, M. Ligueros-Saylan, S. Mudaliar, Hyperglycemia associated with pasireotide: results from a mechanistic study in healthy volunteers. J. Clin. Endocrinol. Metab. 98(8), 3446–3453 (2013)
J. van der Hoek, W.W. de Herder, R.A. Feelders, A.J. van der Lely, P. Uitterlinden, V. Boerlin, C. Bruns, K.W. Poon, I. Lewis, G. Weckbecker, T. Krahnke, L.J. Hofland, S.W. Lamberts, A single-dose comparison of the acute effects between the new somatostatin analog SOM230 and octreotide in acromegalic patients. J. Clin. Endocrinol. Metab. 89(2), 638–645 (2004)
D.L. Batista, X. Zhang, R. Gejman, P.J. Ansell, Y. Zhou, S.A. Johnson, B. Swearingen, E.T. Hedley-Whyte, C.A. Stratakis, A. Klibanski, The effects of SOM230 on cell proliferation and adrenocorticotropin secretion in human corticotroph pituitary adenomas. J. Clin. Endocrinol. Metab. 91, 4482–4488 (2006)
L.J. Hofland, J. van der Hoek, R. Feelders, M.O. van Aken, P.M. van Koetsveld, M. Waaijers, D. Sprij-Mooij, C. Bruns, G. Weckbecker, W.W. de Herder, A. Beckers, S.W. Lamberts, The multi-ligand somatostatin analogue SOM230 inhibits ACTH secretion by cultured human corticotroph adenomas via somatostatin receptor type 5. Eur. J. Endocrinol. 152, 645–654 (2005)
M. Boscaro, J. Bertherat, J. Findling, M. Fleseriu, A.B. Atkinson, S. Petersenn, J. Schopohl, P. Snyder, G. Hughes, A. Trovato, K. Hu, M. Maldonado, B.M. Biller, Extended treatment of Cushing’s disease with pasireotide: results from a 2-year, Phase II study. Pituitary 17(4), 320–326 (2014)
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trementino, L., Zilio, M., Marcelli, G. et al. The role of an acute pasireotide suppression test in predicting response to treatment in patients with Cushing’s disease: findings from a pilot study. Endocrine 50, 154–161 (2015). https://doi.org/10.1007/s12020-014-0499-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-014-0499-0