Abstract
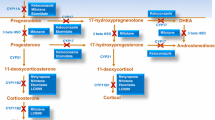
Cushing’s disease (CD) is associated with increased morbidity and mortality. Until now, no medical treatment has been shown to be totally satisfactory when administrated alone. This study aimed to assess the effectiveness of cabergoline with added ketoconazole and of the same combination in reverse, using urinary free cortisol (UFC) and late night salivary cortisol (LNSC) levels as biochemical markers of the treatments’ efficacy in CD patients. A prospective analysis conducted on 14 patients (f/m = 12/2; median age 52, range 33–70 years) divided into two groups: 6 patients initially treated with cabergoline for 4–6 months (rising from 0.5–1 mg/week up to 3.0 mg/week), after which ketoconazole was added (group A); and 8 patients first took ketoconazole alone for 4–6 months (rising from 200 mg/day to 600 mg/day), then cabergoline was added (group B). Patients were compared with 14 age-matched patients in prolonged remission after effective neurosurgery for CD. The combination therapy led to UFC normalization in 79 % of patients with no differences between the groups; only one patient failed to respond at all. Neither drug succeeded in controlling the disease when taken alone. LNSC dropped when compared to baseline levels, but not to a significant degree (p = 0.06), and it remained significantly higher than in controls (p = 0.0006). Associating cabergoline with ketoconazole may represent an effective second-line treatment, achieving a satisfactory reduction in UFC levels and clinical improvement. Although the combined treatment lowered patients’ LNSC levels, they remained higher than normal, indicating a persistent subclinical hypercortisolism; the implications of this condition need to be considered. No differences emerged between the two treatment schedules.



Similar content being viewed by others
References
Patil CG, Prevedello DM, Lad SP, Vance ML, Thorner MO, Katznelson L, Laws ER Jr (2008) Late recurrences of Cushing’s disease after initial successful transsphenoidal surgery. J Clin Endocrinol Metab 93:358–362
Atkinson AB, Kennedy A, Wiggam MI, McCance DR, Sheridan B (2005) Long-term remission rates after pituitary surgery for Cushing’s disease: the need for long-term surveillance. Clin Endocrinol (Oxf) 63:549–559
Rees DA, Hanna FW, Davies JS, Mills RG, Vafidis J, Scanlon MF (2002) Long-term follow-up results of transsphenoidal surgery for Cushing’s disease in a single centre using strict criteria for remission. Clin Endocrinol (Oxf) 56:541–551
Sheehan JM, Lopes MB, Sheehan JP, Ellegala D, Webb KM, Laws ER Jr (2000) Results of transsphenoidal surgery for Cushing’s disease in patients with no histologically confirmed tumor. Neurosurgery 47:33–39
Bochicchio D, Losa M, Buchfelder M (1995) Factors influencing the immediate and late outcome of Cushing’s disease treated by transsphenoidal surgery: a retrospective study by the European Cushing’s Disease Survey Group. J Clin Endocrinol Metab 80:3114–3120
Tritos NA, Biller BM, Swearingen B (2011) Management of Cushing disease. Nat Rev Endocrinol 7:279–289
Sonino N (1987) The use of ketoconazole as an inhibitor of steroid production. N Engl J Med 317:812–818
Feldman D (1986) Ketoconazole and other imidazole derivatives as inhibitors of steroidogenesis. Endocr Rev 7:409–420
Pivonello R, Ferone D, de Herder WW, Kros JM, De Caro ML, Arvigo M, Annunziato L, Lombardi G, Colao A, Hofland LJ, Lamberts SW (2004) Dopamine receptor expression and function in corticotroph pituitary tumors. J Clin Endocrinol Metab 89:2452–2462
Vilar L, Naves LA, Azevedo MF, Arruda MJ, Arahata CM, Moura E, Silva L, Agra R, Pontes L, Montenegro L, Albuquerque JL, Canadas V (2010) Effectiveness of cabergoline in monotherapy and combined with ketoconazole in the management of Cushing’s disease. Pituitary 13:123–129
Biller BMK, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J et al (2008) Treatment of ACTH-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 93:2454–2462
Hoffman BM, Hlavac M, Martinez R, Buchfelder M, Müller OA, Fahlbusch R (2008) Long-term results after microsurgery for Cushing disease: experience with 426 primary operations over 35 years. J Neurosurg 108:9–18
Pivonello R, De Martino MC, Cappabianca P, De Leo M, Faggiano A, Lombardi G, Hofland LJ, Lamberts SW, Colao A (2009) The medical treatment of Cushing’s disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J Clin Endocrinol Metab 94:223–230
Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM (2008) The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 93:1526–1540
Feelders RA, de Bruin C, Pereira AM, Romijn JA, Netea-Maier RT, Hermus AR, Zelissen PM, van Heerebeek R, de Jong FH, van der Lely AJ, de Herder WW, Hofland LJ, Lamberts SW (2010) Pasireotide alone or with cabergoline and ketoconazole in Cushing’s disease. N Engl J Med 362:1846–1848
Boscaro M, Ludlam WH, Atkinson B, Glusman JE, Petersenn S, Reincke M, Snyder P, Tabarin A, Biller BM, Findling J, Melmed S, Darby CH, Hu K, Wang Y, Freda PU, Grossman AB, Frohman LA, Bertherat J (2009) Treatment of pituitary-dependent Cushing’s disease with the multireceptor ligand somatostatin analog pasireotide (SOM230): a multicenter, phase II trial. J Clin Endocrinol Metab 94:115–122
Colao A, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, Schoenherr U, Mills D, Salgado LR, Biller BM, Pasireotide B2305 Study Group (2012) A 12-month phase 3 study of pasireotide in Cushing’s disease. N Engl J Med 366:914–924
Pecori Giraldi F, Ambrogio AG, Andrioli M, Sanguin F, Karamouzis I, Corsello SM, Scaroni C, Arvat E, Pontercorvi A, Cavagnini F (2012) Potential role for retinoic acid in patients with Cushing’s disease. J Clin Endocrinol Metab 97:3577–3583
Páez-Pereda M, Kovalovsky D, Hopfner U, Theodoropoulou M, Pagotto U, Uhl E, Losa M, Stalla J, Grübler Y, Missale C, Arzt E, Stalla GK (2001) Retinoic acid prevents experimental Cushing’s syndrome. J Clin Invest 108:1123–1131
Bevan JS, Webster J, Burke CW, Scanlon MF (1992) Dopamine agonists and pituitary tumor shrinkage. Endocr Rev 13:220–240
Mercado-Asis LB, Yasuda K, Murayama M, Mune T, Morita H, Miura K (1992) Beneficial effects of high daily dose bromocriptine treatment in Cushing’s disease. Endocrinol Jpn 39:385–395
Lamberts SW, Klijn JG, de Quijada M, Timmermans HA, Uitterlinden P, de Jong FH, Birkenhäger JC (1980) The mechanism of the suppressive action of bromocriptine on adrenocorticotropin secretion in patients with Cushing’s disease and Nelson’s syndrome. J Clin Endocrinol Metab 51:307–311
Colao A, Di Sarno A, Marzullo P, Di Somma C, Cerbone G, Landi ML, Faggiano A, Merola B, Lombardi G (2000) New medical approaches in pituitary adenomas. Horm Res 53:76–87
Petrossians P, Thonnard AS, Beckers A (2010) Medical treatment in Cushing’s syndrome: dopamine agonists and cabergoline. Neuroendocrinology 92:116–119
Godbout A, Manavela M, Danilowicz K, Beauregard H, Bruno OD, Lacroix A (2010) Cabergoline monotherapy in the long-term treatment of Cushing’s disease. Eur J Endocrinol 163:709–716
Lila AR, Gopal RA, Acharya SV, George J, Sarathi V, Bandgar T, Menon PS, Shah NS (2010) Efficacy of cabergoline in uncured (persistent or recurrent) Cushing disease after pituitary surgical treatment with or without radiotherapy. Endocr Pract 16:968–976
Engelhardt D, Weber MM, Miksch T, Abedinpour F, Jaspers C (1991) The influence of ketoconazole on human adrenal steroidogenesis: incubation studies with tissue slices. Clin Endocrinol (Oxf) 35:163–168
Sonino N, Boscaro M (1999) Medical therapy for Cushing’s disease. Endocrinol Metab Clin North Am 28:211–222
Sonino N, Boscaro M, Paoletta A, Mantero F, Ziliotto D (1991) Ketoconazole treatment in Cushing’s syndrome: experience in 34 patients. Clin Endocrinol (Oxf) 35:347–352
Castinetti F, Morange I, Jaquet P, Conte-Devolx B, Brue T (2008) Ketoconazole revisited: a preoperative or postoperative treatment in Cushing’s disease. Eur J Endocrinol 158:91–99
de Bruin C, Feelders RA, Waaijers AM, van Koetsveld PM, Sprij-Mooij DM, Lamberts SW, Hofland LJ (2009) Differential regulation of human dopamine D2 and somatostatin receptor subtype expression by glucocorticoids in vitro. J Mol Endocrinol 42:47–56
de Bruin C, Pereira AM, Feelders RA, Romijn JA, Roelfsema F, Sprij-Mooij DM, van Aken MO, van der Lelij AJ, de Herder WW, Lamberts SW, Hofland LJ (2009) Coexpression of dopamine and somatostatin receptor subtypes in corticotroph adenomas. J Clin Endocrinol Metab 94:1118–1124
Carrasco CA, Coste J, Guignat L, Groussin L, Dugué MA, Gaillard S, Bertagna X, Bertherat J (2008) Midnight salivary cortisol determination for assessing the outcome of transsphenoidal surgery in Cushing’s disease. J Clin Endocrinol Metab 93:4728–4734
Raff H (2009) Utility of salivary cortisol measurements in Cushing’s syndrome and adrenal insufficiency. J Clin Endocrinol Metab 94:3647–3655
Nunes ML, Vattaut S, Corcuff JB, Rault A, Loiseau H, Gatta B, Valli N, Letenneur L, Tabarin A (2009) Late-night salivary cortisol for diagnosis of overt and subclinical Cushing’s syndrome in hospitalized and ambulatory patients. J Clin Endocrinol Metab 94:456–462
Bou Khalil R, Baudry C, Guignat L, Carrasco C, Guibourdenche J, Gaillard S, Bertagna X, Bertherat J (2011) Sequential hormonal changes in 21 patients with recurrent Cushing’s disease after successful pituitary surgery. Eur J Endocrinol 165:729–737
van der Pas R, de Bruin C, Pereira AM, Romijn JA, Netea-Maier RT, Hermus AR, Zelissen PM, de Jong FH, van der Lely AJ, de Herder WW, Webb SM, Lamberts SW, Hofland LJ, Feelders RA (2012) Cortisol diurnal rhythm and quality of life after successful medical treatment of Cushing’s disease. Pituitary doi. doi:10.1007/211102-012-0452-2
Murphy MB (2000) Dopamine: a role in the pathogenesis and treatment of hypertension. J Hum Hypertens 14:S47–S50
Ethical standard
The experiment complied with current legislation in our country.
Conflict of interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barbot, M., Albiger, N., Ceccato, F. et al. Combination therapy for Cushing’s disease: effectiveness of two schedules of treatment. Should we start with cabergoline or ketoconazole?. Pituitary 17, 109–117 (2014). https://doi.org/10.1007/s11102-013-0475-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-013-0475-3