Abstract
Irreversible myocardium infarction is one of the leading causes of cardiovascular disease (CVD) related death and its quantum is expected to grow in coming years. Pharmacological intervention has been at the forefront to ameliorate injury-related morbidity and mortality. However, its outcomes are highly skewed. As an alternative, stem cell-based tissue engineering/regenerative medicine has been explored quite extensively to regenerate the damaged myocardium. The therapeutic modality that has been most widely studied both preclinically and clinically is based on adult multipotent mesenchymal stem cells (MSC) delivered to the injured heart. However, there is debate over the mechanistic therapeutic role of MSC in generating functional beating cardiomyocytes. This review intends to emphasize the role and use of MSC in cardiac regenerative therapy (CRT). We have elucidated in detail, the various aspects related to the history and progress of MSC use in cardiac tissue engineering and its multiple strategies to drive cardiomyogenesis. We have further discussed with a focus on the various therapeutic mechanism uncovered in recent times that has a significant role in ameliorating heart-related problems. We reviewed recent and advanced technologies using MSC to develop/create tissue construct for use in cardiac regenerative therapy. Finally, we have provided the latest update on the usage of MSC in clinical trials and discussed the outcome of such studies in realizing the full potential of MSC use in clinical management of cardiac injury as a cellular therapy module.
Graphical abstract




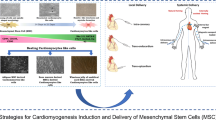
reproduced with permission from Ref [38]). (B) Effect of HGF expressing MSC in cardio myogenesis and improvement in infarcted heart condition. (i) study design for cell loaded patch preparation (ii) 3D Printing of MSC loaded cells on a PCL framework. (iii) 3D printed patch (iv) Transplantation in a rat model of myocardium infarction, (v) isolated heart from patch transplanted in rats (reproduced with permission from Ref [39]), (C) Role of small molecule on improving the differentiation potential and efficiency of MSC into cardiomyocyte like cells. (i) Morphology of MSC when cultured with CHIR for 30 days, (ii) Positive staining of CHIR treated MSC-differentiated towards cardiomyocyte linage expressing cardiomyocyte-specific protein marker.; (ii) Enhanced gene expression of cardiomyocyte like gene from CHIR treated MSC as compared to untreated MSC. (reproduced with permission from Ref [40]); (D) Cardiomyogenic differentiation of MSC on cardiac fibroblast derived extracellular matrix (cardiogel). (i) and (ii) differentiation on tissue culture plates without and with 5Aza treatment in the absence of cardiogel respectively, (iii) and (iv) cardiomyocyte like cells derivation from MSC when cultured on cardio gel without and with 5 Aza treatment respectively (reproduced with permission from Ref [41])

reproduced with permission from Ref [69]); (B) Differentiation of MSC into cardiomyocytes like cells when cultured on nanopatterned substrate. (i) nanopattern plates (ii) shapes and morphology of nanopattern used for MSC culture and differentiation, (iii) morphology of MSC upon differentiation to cardiomyocytes like cells due to nontopographical cues. (reproduced with permission from Ref [70]); (C) Effect of mechanical cyclical stretching on induction of cardio myogenesis in MSC. (i) Set up used for subjecting cultured MSC on silicon to cyclical stretching (ii) diagrammatic representation of the setup, (iii) MSC undergoing change in morphology and alignment and staining possible for the cardiomyocytes marker, (iv) Increased cardiomyocytes specific gene expression of MSC under cyclical stretching (v) cells showing positive staining for cardiomyocytes specific marker (reproduced with permission from Ref [71]); (D) Effect of electrical stimulation on cardiomyogenic induction of MSC. MSC cultured on carbon nanotube-poly Lactic acid nanofiber showed positive staining for cardiomyocytes specific protein marker (reproduced with permission from Ref [22])


reproduced with permission from Ref [93]). (B). Autophagy induced by MSC-derived exosomes decreases CM apoptosis. B(i) Autophagy protein detection. B(ii) CM apoptosis measured using TUNEL assay. B(iii) MI size detected by staining. (reproduced with permission from Ref [94]). (C). Effect of integrin-linked kinase (ILK)-MSC conditioned medium on MI size and fibrosis. C(i) Infarct size in different experiment groups with Masson Trichrome staining. C(ii) Quantification of stained myocardial area (reproduced with permission from Ref [95]). (D). MSCs stimulate endogenous cardiac stem cells. D(i) Contribution of CM precursors following exogenous administration of MSCs. D(ii) 2 weeks the number of C-kit + cells co-expressing GATA-4 D(iii-iv) Chimeric myocardium contains CM (open arrow), MSCs (arrowheads, inset) and cardiac precursors of MSCs origin (arrow), coupled by connexin-43. D(v) Cluster of c-kit + CSCs in an MSCs-treated heart. D (vi) c-kit + cells found in non-MSC treated animals (reproduced with permission from ref [96]). (E). MSC-conditioned media suppresses ROS generation under hypoxic conditions. E(i) Fluorescence images corresponding to ROS suppression. (reproduced with permission from Ref [97]). (F). miR-486-5p from exosomes derived from MSC inhibit apoptosis of CM. F(i) Cell viability ratio of H9C2 cells treated with different mi-BMSC-exosomes. F(ii-iii) Flow cytometry analysis. (reproduced with permission from Ref [98]). (G). Exosomes derived from MSCs promote angiogenesis. G(i) Representative images of tube-like structures and quantitation of the total tube length in control and exosome treated groups. G(ii) Gross look of matrigel plugs. G(iii) Haemoglobin content. G(iv) CD31 staining and quantification of the CD31-positive cells (reproduced with permission from Ref [99])


reproduced with permission from Ref [194]). (B). Microfluidic device (MD) for directional migration of MSCs. B(i) The MD set up. B(ii) MSC extravasation and migration in MD. B(iii) Endothelial monolayers and MSC migration due to SDF-1α gradient. B(iv) MSC extravasation (reproduced with permission from Ref [152]). (C). Cell sheet technology. C(i) The thermoresponsive culture surface supports cell adhesion and detachment. C(ii) Magneto-fluorescent stem cell sheet. C(iii) 3D reconstitution of cell sheet(reproduced with permission from Ref [198]). (D). Dual approach improves cardiac function following MI. D(i) Cardiac function in rats receiving cardiomyocytes derived from human iPSC with human MSC cell-loaded patch. D(ii) Images of stained capillaries 8 weeks after MI. D (iii) Representative images from the experimental groups showing cardiac fibrosis after staining with Masson’s trichrome 8 weeks after MI (reproduced with permission from Ref [199]). (E). Electrospinning as a tool for hMSC application. E(i) Nanofiber scaffolds based on polyvinyl alcohol, chitosan and carbon nanotubes for differentiation of hMSC (reproduced with permission from Ref [200])
Similar content being viewed by others
Data Availability
Data that support the findings of this study are available in the manuscript.
Abbreviations
- MSCs:
-
Mesenchymal Stem Cells
- hMSCs:
-
human Mesenchymal Stem Cells
- BM MSC:
-
Bone Marrow Mesenchymal Stem Cells
- hUC-MSCs:
-
Human Umbilical Cord Mesenchymal Stem Cells
- CLC:
-
Cardiomyocyte Like Cells
- CPC:
-
Cardiac Progenitor Cells
- CSCs:
-
Cardiac Stem Cells
- hiPSC:
-
Human induced Pluripotent Stem Cells
- CRT:
-
Cardiac Regenerative Therapy
- CVD:
-
Cardiovascular Disease
- BMP:
-
Bone Morphogenetic Protein
- VEGF:
-
Vascular Endothelial Growth Factor
- TGF-β1:
-
Transforming Growth Factor Beta1
- FGF:
-
Fibroblast Growth Factor
- PDGF:
-
Platelet Derived Growth Factor
- HGF:
-
Hepatocytes Growth Factor
- GSK-3β:
-
Glycogen Synthase 3 beta
- MHC:
-
Major Histocompatibility Complex
- 5Aza:
-
5 Azacytidine
- ECM:
-
Extracellular Matrix
- miRNA:
-
Micro RNA
- HDAC:
-
Histone Deacetylase Inhibitor
- GATA4:
-
GATA-binding factor 4
- Nkx2.5:
-
Homeobox Protein Nkx-2.5
- CMHC:
-
Cardiac Myosin Heavy Chain
- cTnI:
-
Cardiac Troponin
- C43:
-
Connexin43
- EVs:
-
Extracellular Vesicles
- CNT:
-
Carbon Nanotube
- 3D Printing:
-
3-Dimensional Printing
- CMC:
-
Cardiac Mesenchymal Cells
References
G.A. Roth, M.D. Huffman, A.E. Moran, V. Feigin, G.A. Mensah, M. Naghavi, C.J.L. Murray, Global and regional patterns in cardiovascular mortality from 1990 to 2013, Circulation. 132 (2015) 1667–1678. https://doi.org/10.1161/CIRCULATIONAHA.114.008720.
Prabhakaran, D., Jeemon, P., & Roy, A. (2016). Cardiovascular diseases in india: current epidemiology and future directions. Circulation, 133, 1605–1620. https://doi.org/10.1161/CIRCULATIONAHA.114.008729
E.J. Benjamin, M.J. Blaha, S.E. Chiuve, M. Cushman, S.R. Das, R. Deo, S.D. De Ferranti, J. Floyd, M. Fornage, C. Gillespie, C.R. Isasi, M.C. Jim’nez, L.C. Jordan, S.E. Judd, D. Lackland, J.H. Lichtman, L. Lisabeth, S. Liu, C.T. Longenecker, R.H. MacKey, K. Matsushita, D. Mozaffarian, M.E. Mussolino, K. Nasir, R.W. Neumar, L. Palaniappan, D.K. Pandey, R.R. Thiagarajan, M.J. Reeves, M. Ritchey, C.J. Rodriguez, G.A. Roth, W.D. Rosamond, C. Sasson, A. Towfghi, C.W. Tsao, M.B. Turner, S.S. Virani, J.H. Voeks, J.Z. Willey, J.T. Wilkins, J.H. Wu, H.M. Alger, S.S. Wong, P. Muntner, Heart Disease and Stroke Statistics’2017 Update: A Report from the American Heart Association, Circulation. 135 (2017) e146–e603. https://doi.org/10.1161/CIR.0000000000000485.
A. Timmis, N. Townsend, C. Gale, R. Grobbee, N. Maniadakis, M. Flather, E. Wilkins, L. Wright, R. Vos, J. Bax, M. Blum, F. Pinto, P. Vardas, European Society of Cardiology: Cardiovascular disease statistics 2017, Eur. Heart J. 39 (2018) 508–577. https://doi.org/10.1093/eurheartj/ehx628.
Feric, N. T., & Radisic, M. (2016). Maturing human pluripotent stem cell-derived cardiomyocytes in human engineered cardiac tissues. Advanced Drug Delivery Reviews, 96, 110–134. https://doi.org/10.1016/j.addr.2015.04.019
Gupta, S., Sharma, A., & Verma, R. S. (2020). Polymers in biosensor devices for cardiovascular applications. Curr. Opin. Biomed. Eng. https://doi.org/10.1016/j.cobme.2019.10.002
Vunjak-Novakovic, G., Tandon, N., Godier, A., Maidhof, R., Marsano, A., Martens, T. P., & Radisic, M. (2010). Challenges in cardiac tissue engineering. Tissue Engineering. Part B, Reviews, 16, 169–187. https://doi.org/10.1089/ten.teb.2009.0352
Bulluck, H., Yellon, D. M., & Hausenloy, D. J. (2016). Reducing myocardial infarct size: Challenges and future opportunities. Heart, 102, 341–348. https://doi.org/10.1136/heartjnl-2015-307855
Cahill, T. J., Choudhury, R. P., & Riley, P. R. (2017). Heart regeneration and repair after myocardial infarction: Translational opportunities for novel therapeutics. Nature Reviews. Drug Discovery, 16, 699–717. https://doi.org/10.1038/nrd.2017.106
Steinhauser, M. L., & Lee, R. T. (2011). Regeneration of the heart. EMBO Molecular Medicine, 3, 701–712. https://doi.org/10.1002/emmm.201100175
P. Hernigou, Bone transplantation and tissue engineering, part IV. Mesenchymal stem cells: history in orthopedic surgery from Cohnheim and Goujon to the Nobel Prize of Yamanaka, Int. Orthop. 2015 394. 39 (2015) 807–817. https://doi.org/10.1007/S00264-015-2716-8.
Friedenstein, A. J., Chailakhjan, R. K., & Lalykina, K. S. (1970). THE DEVELOPMENT OF FIBROBLAST COLONIES IN MONOLAYER CULTURES OF GUINEA-PIG BONE MARROW AND SPLEEN CELLS. Cell Proliferation, 3, 393–403. https://doi.org/10.1111/j.1365-2184.1970.tb00347.x
A. Biochem, E./ Biotechnol, J.W. Kuhbier, B. Weyand, C. Radtke, P.M. Vogt, C. Kasper, K. Reimers, Isolation, Characterization, Differentiation, and Application of Adipose-Derived Stem Cells, (n.d.). https://doi.org/10.1007/10_2009_24.
Noiseux, N., Gnecchi, M., Lopez-Ilasaca, M., Zhang, L., Solomon, S. D., Deb, A., Dzau, V. J., & Pratt, R. E. (2006). Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Molecular Therapy, 14, 840–850. https://doi.org/10.1016/j.ymthe.2006.05.016
Z. X, B. H, C. CY, Y. L, F. D, H. BS, C. B, E. E, Electrospun fine-textured scaffolds for heart tissue constructs, Biomaterials. 26 (2005) 5330–5338. https://doi.org/10.1016/J.BIOMATERIALS.2005.01.052.
Z. WH, M. I, W. G, D. M, N. H, N. U, H. A, B. L, B. K, M. B, D. S, S. A, E. H, E. T, Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts, Nat. Med. 12 (2006) 452–458. https://doi.org/10.1038/NM1394.
G. J, L. GS, B. CY, H. ZM, H. MY, Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction, Inflammation. 30 (2007) 97–104. https://doi.org/10.1007/S10753-007-9025-3.
R. Mazhari, J.M. Hare, Mechanisms of action of mesenchymal stem cells in cardiac repair: potential influences on the cardiac stem cell niche, Nat. Clin. Pract. Cardiovasc. Med. 2007 41. 4 (2007) S21–S26. https://doi.org/10.1038/ncpcardio0770.
Mc, Y., Ss, W., Nk, C., Nh, C., Yy, H., Yl, C., Mj, S., & Tw, C. (2009). The cardiomyogenic differentiation of rat mesenchymal stem cells on silk fibroin-polysaccharide cardiac patches in vitro. Biomaterials, 30, 3757–3765. https://doi.org/10.1016/J.BIOMATERIALS.2009.03.057
G. D, L. X, L. L, W. J, T. Q, S. Y, C. H, Chemical and physical stimuli induce cardiomyocyte differentiation from stem cells, Biochem. Biophys. Res. Commun. 381 (2009) 317–321. https://doi.org/10.1016/J.BBRC.2009.01.173.
G. R, M. N, L. J, G. J, K. L, K. C, G. M, T. A, W. W, M. P, W. F, C. B, L. W, S. G, Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration, Biomaterials. 32 (2011) 9218–9230. https://doi.org/10.1016/J.BIOMATERIALS.2011.08.071.
Mooney, E., Mackle, J. N., Blond, D. J. P., O’Cearbhaill, E., Shaw, G., Blau, W. J., Barry, F. P., Barron, V., & Murphy, J. M. (2012). The electrical stimulation of carbon nanotubes to provide a cardiomimetic cue to MSCs. Biomaterials, 33, 6132–6139. https://doi.org/10.1016/j.biomaterials.2012.05.032
Ai, C. (1991). Mesenchymal stem cells. Journal of Orthopaedic Research, 9, 641–650. https://doi.org/10.1002/JOR.1100090504
Liu, C., Fan, Y., Zhou, L., Zhu, H. Y., Song, Y. C., Hu, L., Wang, Y., & Li, Q. P. (2015). Pretreatment of mesenchymal stem cells with angiotensin II enhances paracrine effects, angiogenesis, gap junction formation and therapeutic efficacy for myocardial infarction. International Journal of Cardiology, 188, 22–32. https://doi.org/10.1016/j.ijcard.2015.03.425
Mayourian, J., Savizky, R. M., Sobie, E. A., & Costa, K. D. (2016). Modeling Electrophysiological Coupling and Fusion between Human Mesenchymal Stem Cells and Cardiomyocytes. PLoS Computational Biology, 12, e1005014. https://doi.org/10.1371/JOURNAL.PCBI.1005014
W. S, S. T, C. AI, Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine, Muscle Nerve. 18 (1995) 1417–1426. https://doi.org/10.1002/MUS.880181212.
Makino, S., Fukuda, K., Miyoshi, S., Konishi, F., Kodama, H., Pan, J., Sano, M., Takahashi, T., Hori, S., Abe, H., Hata, J. I., Umezawa, A., & Ogawa, S. (1999). Cardiomyocytes can be generated from marrow stromal cells in vitro. The Journal of Clinical Investigation, 103, 697–705. https://doi.org/10.1172/JCI5298
F. S, B. R, Z. YF, S. M, P. A, T. FO, W. NJ, L. MB, E. SE, K. R, Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia, J. Am. Coll. Cardiol. 37 (2001) 1726–1732. https://doi.org/10.1016/S0735-1097(01)01200-1.
Toma, C., Pittenger, M. F., Cahill, K. S., Byrne, B. J., & Kessler, P. D. (2002). Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation, 105, 93–98. https://doi.org/10.1161/hc0102.101442
S. JG, G. PJ, B. WA, S. G, M. J, R. JM, P. MF, M. BJ, Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects, Ann. Thorac. Surg. 73 (2002) 1919–1926. https://doi.org/10.1016/S0003-4975(02)03517-8.
R. S, E. JW, W. AS, K. JY, Cardiomyocyte-mediated contact programs human mesenchymal stem cells to express cardiogenic phenotype, J. Thorac. Cardiovasc. Surg. 126 (2003) 124–132. https://doi.org/10.1016/S0022-5223(03)00074-6.
K. M, W. Y, W. MA, X. M, A. A, A. M, Implantation of bone marrow stem cells reduces the infarction and fibrosis in ischemic mouse heart, J. Mol. Cell. Cardiol. 35 (2003) 1113–1119. https://doi.org/10.1016/S0022-2828(03)00211-6.
M. Cui, Z. Wang, R. Bassel-Duby, E.N. Olson, Genetic and epigenetic regulation of cardiomyocytes in development, regeneration and disease, Dev. 145 (2018). https://doi.org/10.1242/dev.171983.
Wamstad, J. A., Alexander, J. M., Truty, R. M., Shrikumar, A., Li, F., Eilertson, K. E., Ding, H., Wylie, J. N., Pico, A. R., Capra, J. A., Erwin, G., Kattman, S. J., Keller, G. M., Srivastava, D., Levine, S. S., Pollard, K. S., Holloway, A. K., Boyer, L. A., & Bruneau, B. G. (2012). Dynamic and coordinated epigenetic regulation of developmental transitions in the cardiac lineage. Cell, 151, 206–220. https://doi.org/10.1016/j.cell.2012.07.035
K. Oyama, D. El-Nachef, Y. Zhang, P. Sdek, W.R. MacLellan, Epigenetic regulation of cardiac myocyte differentiation, Front. Genet. 5 (2014). https://doi.org/10.3389/fgene.2014.00375.
Perez-Campo, F., & Riancho, J. (2015). Epigenetic Mechanisms Regulating Mesenchymal Stem Cell Differentiation. Current Genomics, 16, 368–383. https://doi.org/10.2174/1389202916666150817202559
Burlacu, A. (2006). Can 5-azacytidine convert the adult stem cells into cardiomyocytes? A brief overview. Archives of Physiology and Biochemistry, 112, 260–264. https://doi.org/10.1080/13813450601094631
Govarthanan, K., Gupta, P. K., Ramasamy, D., Kumar, P., Mahadevan, S., & Verma, R. S. (2020). DNA methylation microarray uncovers a permissive methylome for cardiomyocyte differentiation in human mesenchymal stem cells. Genomics, 112, 1384–1395. https://doi.org/10.1016/j.ygeno.2019.08.007
B.W. Park, S.H. Jung, S. Das, S.M. Lee, J.H. Park, H. Kim, J.W. Hwang, S. Lee, H.J. Kim, H.Y. Kim, S. Jung, D.W. Cho, J. Jang, K. Ban, H.J. Park, In vivo priming of human mesenchymal stem cells with hepatocyte growth factor–engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair, Sci. Adv. 6 (2020). https://doi.org/10.1126/sciadv.aay6994.
Govarthanan, K., Vidyasekar, P., Gupta, P. K., Lenka, N., & Verma, R. S. (2020). Glycogen synthase kinase 3β inhibitor- CHIR 99021 augments the differentiation potential of mesenchymal stem cells. Cytotherapy, 22, 91–105. https://doi.org/10.1016/j.jcyt.2019.12.007
P. Sreejit, R.S. Verma, Cardiogel supports adhesion, proliferation and differentiation of stem cells with increased oxidative stress protection, Eur. Cells Mater. 21 (2011) 107–121. https://doi.org/10.22203/eCM.v021a09.
S. Gupta, A. Sharma, A. S, R.S. Verma, Mesenchymal Stem Cells for Cardiac Regeneration: from Differentiation to Cell Delivery, Stem Cell Rev. Reports 2021 175. 17 (2021) 1666–1694. https://doi.org/10.1007/S12015-021-10168-0.
H. Shen, Y. Wang, Z. Zhang, J. Yang, S. Hu, Z. Shen, Mesenchymal Stem Cells for Cardiac Regenerative Therapy: Optimization of Cell Differentiation Strategy, Stem Cells Int. 2015 (2015). https://doi.org/10.1155/2015/524756.
Kuraitis, D., Ruel, M., & Suuronen, E. J. (2011). Mesenchymal stem cells for cardiovascular regeneration. Cardiovascular Drugs and Therapy, 25, 349–362. https://doi.org/10.1007/s10557-011-6311-y
R.S. Verma, Recent Advances in Induced Pluripotent Stem Cell (iPSC) based Therapeutics, J. Stem Cell Res. Ther. 3 (2017). https://doi.org/10.15406/jsrt.2017.03.00100.
X. Guo, Y. Bai, L. Zhang, B. Zhang, N. Zagidullin, K. Carvalho, Z. Du, B. Cai, Cardiomyocyte differentiation of mesenchymal stem cells from bone marrow: New regulators and its implications, Stem Cell Res. Ther. 9 (2018). https://doi.org/10.1186/s13287-018-0773-9.
Y. Guo, Y. Yu, S. Hu, Y. Chen, Z. Shen, The therapeutic potential of mesenchymal stem cells for cardiovascular diseases, Cell Death Dis. 11 (2020). https://doi.org/10.1038/s41419-020-2542-9.
L.C. Liew, B.X. Ho, B.S. Soh, Mending a broken heart: Current strategies and limitations of cell-based therapy, Stem Cell Res. Ther. 11 (2020). https://doi.org/10.1186/s13287-020-01648-0.
Song, H., Chang, W., Song, B. W., & Hwang, K. C. (2012). Specific differentiation of mesenchymal stem cells by small molecules. Am. J. Stem Cells., 1, 22–30.
B. Huang, G. Li, X.H. Jiang, Fate determination in mesenchymal stem cells: A perspective from histone-modifying enzymes, Stem Cell Res. Ther. 6 (2015). https://doi.org/10.1186/s13287-015-0018-0.
R. Santhakumar, P. Vidyasekar, R.S. Verma, Cardiogel: A nano-matrix scaffold with potential application in cardiac regeneration using mesenchymal stem cells, PLoS One. 9 (2014). https://doi.org/10.1371/journal.pone.0114697.
X. Shen, B. Pan, H. Zhou, L. Liu, T. Lv, J. Zhu, X. Huang, J. Tian, Differentiation of mesenchymal stem cells into cardiomyocytes is regulated by miRNA-1–2 via WNT signaling pathway, J. Biomed. Sci. 24 (2017). https://doi.org/10.1186/s12929-017-0337-9.
Cai, B., Li, J., Wang, J., Luo, X., Ai, J., Liu, Y., Wang, N., Liang, H., Zhang, M., Chen, N., Wang, G., Xing, S., Zhou, X., Yang, B., Wang, X., & Lu, Y. (2012). MicroRNA-124 regulates cardiomyocyte differentiation of bone marrow-derived mesenchymal stem cells via targeting STAT3 signaling. Stem Cells., 30, 1746–1755. https://doi.org/10.1002/stem.1154
Ng, W. H., Ramasamy, R., Yong, Y. K., Ngalim, S. H., Lim, V., Shaharuddin, B., & Tan, J. J. (2019). Extracellular matrix from decellularized mesenchymal stem cells improves cardiac gene expressions and oxidative resistance in cardiac C-kit cells. Regen. Ther., 11, 8–16. https://doi.org/10.1016/j.reth.2019.03.006
Ott, H. C., Matthiesen, T. S., Goh, S. K., Black, L. D., Kren, S. M., Netoff, T. I., & Taylor, D. A. (2008). Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nature Medicine, 14, 213–221. https://doi.org/10.1038/nm1684
Akbay, E., & Onur, M. A. (2019). Investigation of survival and migration potential of differentiated cardiomyocytes transplanted with decellularized heart scaffold. J. Biomed. Mater. Res. - Part A., 107, 561–570. https://doi.org/10.1002/jbm.a.36572
C. Tong, C. Li, B. Xie, M. Li, X. Li, Z. Qi, J. Xia, Generation of bioartificial hearts using decellularized scaffolds and mixed cells, Biomed. Eng. Online. 18 (2019). https://doi.org/10.1186/s12938-019-0691-9.
M. Shah, P. Kc, K.M. Copeland, J. Liao, G. Zhang, A Thin Layer of Decellularized Porcine Myocardium for Cell Delivery, Sci. Rep. 8 (2018). https://doi.org/10.1038/s41598-018-33946-2.
R. Bai, L. Tian, Y. Li, J. Zhang, Y. Wei, Z. Jin, Z. Liu, H. Liu, Combining ECM Hydrogels of Cardiac Bioactivity with Stem Cells of High Cardiomyogenic Potential for Myocardial Repair, Stem Cells Int. 2019 (2019). https://doi.org/10.1155/2019/6708435.
Reis, L. A., Chiu, L. L. Y., Feric, N., Fu, L., & Radisic, M. (2016). Biomaterials in myocardial tissue engineering. Journal of Tissue Engineering and Regenerative Medicine, 10, 11–28. https://doi.org/10.1002/term.1944
Nasr, S. M., Rabiee, N., Hajebi, S., Ahmadi, S., Fatahi, Y., Hosseini, M., Bagherzadeh, M., Ghadiri, A. M., Rabiee, M., Jajarmi, V., & Webster, T. J. (2020). Biodegradable nanopolymers in cardiac tissue engineering: From concept towards nanomedicine. International Journal of Nanomedicine, 15, 4205–4224. https://doi.org/10.2147/IJN.S245936
Pascual-Gil, S., Garbayo, E., Díaz-Herráez, P., Prosper, F., & Blanco-Prieto, M. J. (2015). Heart regeneration after myocardial infarction using synthetic biomaterials. Journal of Controlled Release, 203, 23–38. https://doi.org/10.1016/j.jconrel.2015.02.009
Kang, P. L., Chen, C. H., Chen, S. Y., Wu, Y. J., Lin, C. Y., Lin, F. H., & Kuo, S. M. (2013). Nano-sized collagen i molecules enhanced the differentiation of rat mesenchymal stem cells into cardiomyocytes. J. Biomed. Mater. Res. - Part A., 101, 2808–2816. https://doi.org/10.1002/jbm.a.34589
Lin, Y. L., Chen, C. P., Lo, C. M., & Wang, H. S. (2016). Stiffness-controlled three-dimensional collagen scaffolds for differentiation of human Wharton’s jelly mesenchymal stem cells into cardiac progenitor cells. J. Biomed. Mater. Res. - Part A., 104, 2234–2242. https://doi.org/10.1002/jbm.a.35762
Wang, H., Shi, J., Wang, Y., Yin, Y., Wang, L., Liu, J., Liu, Z., Duan, C., Zhu, P., & Wang, C. (2014). Promotion of cardiac differentiation of brown adipose derived stem cells by chitosan hydrogel for repair after myocardial infarction. Biomaterials, 35, 3986–3998. https://doi.org/10.1016/j.biomaterials.2014.01.021
Liu, B. H., Yeh, H. Y., Lin, Y. C., Wang, M. H., Chen, D. C., Lee, B. H., & Hsu, S. H. (2013). Spheroid formation and enhanced cardiomyogenic potential of adipose-derived stem cells grown on chitosan, Biores. Open. Access, 2, 28–39. https://doi.org/10.1089/biores.2012.0285
Lee, W. C., Lim, C. H. Y. X., Shi, H., Tang, L. A. L., Wang, Y., Lim, C. T., & Loh, K. P. (2011). Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano, 5, 7334–7341. https://doi.org/10.1021/nn202190c
Park, J., Park, S., Ryu, S., Bhang, S. H., Kim, J., Yoon, J. K., Park, Y. H., Cho, S. P., Lee, S., Hong, B. H., & Kim, B. S. (2014). Graphene-regulated cardiomyogenic differentiation process of mesenchymal stem cells by enhancing the expression of extracellular matrix proteins and cell signaling molecules. Adv. Healthc. Mater., 3, 176–181. https://doi.org/10.1002/adhm.201300177
Park, J., Kim, Y. S., Ryu, S., Kang, W. S., Park, S., Han, J., Jeong, H. C., Hong, B. H., Ahn, Y., & Kim, B. S. (2015). Graphene potentiates the myocardial repair efficacy of mesenchymal stem cells by stimulating the expression of angiogenic growth factors and gap junction protein. Advanced Functional Materials, 25, 2590–2600. https://doi.org/10.1002/adfm.201500365
Seo, H. R., Joo, H. J., Kim, D. H., Cui, L. H., Choi, S. C., Kim, J. H., Cho, S. W., Lee, K. B., & Lim, D. S. (2017). Nanopillar Surface Topology Promotes Cardiomyocyte Differentiation through Cofilin-Mediated Cytoskeleton Rearrangement. ACS Applied Materials & Interfaces, 9, 16803–16812. https://doi.org/10.1021/acsami.7b01555
Y. Huang, L. Zheng, X. Gong, X. Jia, W. Song, M. Liu, Y. Fan, Effect of cyclic strain on cardiomyogenic differentiation of rat bone marrow derived mesenchymal stem cells, PLoS One. 7 (2012). https://doi.org/10.1371/journal.pone.0034960.
Huang, J., Chen, Y., Tang, C., Fei, Y., Wu, H., Ruan, D., Paul, M. E., Chen, X., Yin, Z., Heng, B. C., Chen, W., & Shen, W. (2019). The relationship between substrate topography and stem cell differentiation in the musculoskeletal system. Cellular and Molecular Life Sciences, 76, 505–521. https://doi.org/10.1007/s00018-018-2945-2
N. Thavandiran, S.S. Nunes, Y. Xiao, M. Radisic, Topological and electrical control of cardiac differentiation and assembly, Stem Cell Res. Ther. 4 (2013). https://doi.org/10.1186/scrt162.
K. Henderson, A.D. Sligar, V.P. Le, J. Lee, A.B. Baker, Biomechanical Regulation of Mesenchymal Stem Cells for Cardiovascular Tissue Engineering, Adv. Healthc. Mater. 6 (2017). https://doi.org/10.1002/adhm.201700556.
R.J. McMurray, A.K.T. Wann, C.L. Thompson, J.T. Connelly, M.M. Knight, Surface topography regulates wnt signaling through control of primary cilia structure in mesenchymal stem cells, Sci. Rep. 3 (2013). https://doi.org/10.1038/srep03545.
Tummala, P., Arnsdorf, E. J., & Jacobs, C. R. (2010). The role of primary cilia in mesenchymal stem cell differentiation: A pivotal switch in guiding lineage commitment. Cellular and Molecular Bioengineering, 3, 207–212. https://doi.org/10.1007/s12195-010-0127-x
Joshi, J., Brennan, D., Beachley, V., & Kothapalli, C. R. (2018). Cardiomyogenic differentiation of human bone marrow-derived mesenchymal stem cell spheroids within electrospun collagen nanofiber mats. J. Biomed. Mater. Res. - Part A., 106, 3303–3312. https://doi.org/10.1002/jbm.a.36530
Yamada, K., Green, K. G., Samarel, A. M., & Saffitz, J. E. (2005). Distinct pathways regulate expression of cardiac electrical and mechanical junction proteins in response to stretch. Circulation Research, 97, 346–353. https://doi.org/10.1161/01.RES.0000178788.76568.8a
Maul, T. M., Chew, D. W., Nieponice, A., & Vorp, D. A. (2011). Mechanical stimuli differentially control stem cell behavior: Morphology, proliferation, and differentiation. Biomechanics and Modeling in Mechanobiology, 10, 939–953. https://doi.org/10.1007/s10237-010-0285-8
Jacot, J. G., Martin, J. C., & Hunt, D. L. (2010). Mechanobiology of cardiomyocyte development. Journal of Biomechanics, 43, 93–98. https://doi.org/10.1016/j.jbiomech.2009.09.014
Guan, J., Wang, F., Li, Z., Chen, J., Guo, X., Liao, J., & Moldovan, N. I. (2011). The stimulation of the cardiac differentiation of mesenchymal stem cells in tissue constructs that mimic myocardium structure and biomechanics. Biomaterials, 32, 5568–5580. https://doi.org/10.1016/j.biomaterials.2011.04.038
B. Wang, G. Wang, F. To, J.R. Butler, A. Claude, R.M. McLaughlin, L.N. Williams, A.L. De Jongh Curry, J. Liao, Myocardial scaffold-based cardiac tissue engineering: Application of coordinated mechanical and electrical stimulations, Langmuir. 29 (2013) 11109–11117. https://doi.org/10.1021/la401702w.
R.S.R.M. Martherus, S.J.V. Vanherle, E.D.J. Timmer, V.A. Zeijlemaker, J.L. Broers, H.J. Smeets, J.P. Geraedts, T.A.Y. Ayoubi, Electrical signals affect the cardiomyocyte transcriptome independently of contraction, Physiol. Genomics. 42 A (2010) 283–289. https://doi.org/10.1152/physiolgenomics.00182.2009.
A. Orza, O. Soritau, L. Olenic, M. Diudea, A. Florea, D. Rus Ciuca, C. Mihu, D. Casciano, A.S. Biris, Electrically conductive gold-coated collagen nanofibers for placental-derived mesenchymal stem cells enhanced differentiation and proliferation, ACS Nano. 5 (2011) 4490–4503. https://doi.org/10.1021/nn1035312.
Caplan, A. I. (2017). Mesenchymal stem cells: Time to change the name! Stem Cells Translational Medicine, 6, 1445–1451. https://doi.org/10.1002/sctm.17-0051
Caplan, A. I. (2019). Medicinal signalling cells: They work, so use them. Nature, 566, 39. https://doi.org/10.1038/D41586-019-00490-6
S.T. Ji, H. Kim, J. Yun, J.S. Chung, S.M. Kwon, Promising Therapeutic Strategies for Mesenchymal Stem Cell-Based Cardiovascular Regeneration: From Cell Priming to Tissue Engineering, Stem Cells Int. 2017 (2017). https://doi.org/10.1155/2017/3945403.
Cho, H. M., Kim, P. H., Chang, H. K., Shen, Y. M., Bonsra, K., Kang, B. J., Yum, S. Y., Kim, J. H., Lee, S. Y., Choi, M. C., Kim, H. H., Jang, G., & Cho, J. Y. (2017). Targeted genome engineering to control VEGF expression in human umbilical cord blood-derived mesenchymal stem cells: Potential implications for the treatment of myocardial infarction. Stem Cells Translational Medicine, 6, 1040–1051. https://doi.org/10.1002/sctm.16-0114
Shibuya, M. (2013). Vascular endothelial growth factor and its receptor system: Physiological functions in angiogenesis and pathological roles in various diseases. Journal of Biochemistry, 153, 13–19. https://doi.org/10.1093/jb/mvs136
Wang, S., Mo, M., Wang, J., Sadia, S., Shi, B., Fu, X., Yu, L., Tredget, E. E., & Wu, Y. (2018). Platelet-derived growth factor receptor beta identifies mesenchymal stem cells with enhanced engraftment to tissue injury and pro-angiogenic property. Cellular and Molecular Life Sciences, 75, 547–561. https://doi.org/10.1007/s00018-017-2641-7
Cheng, M., Huang, K., Zhou, J., Yan, D., Tang, Y. L., Zhao, T. C., Miller, R. J., Kishore, R., Losordo, D. W., & Qin, G. (2015). A critical role of Src family kinase in SDF-1/CXCR4-mediated bone-marrow progenitor cell recruitment to the ischemic heart. Journal of Molecular and Cellular Cardiology, 81, 49–53. https://doi.org/10.1016/j.yjmcc.2015.01.024
G. Gómez-Mauricio, I. Moscoso, M.F. Martín-Cancho, V. Crisóstomo, C. Prat-Vidal, C. Báez-Díaz, F.M. Sánchez-Margallo, A. Bernad, Combined administration of mesenchymal stem cells overexpressing IGF-1 and HGF enhances neovascularization but moderately improves cardiac regeneration in a porcine model, Stem Cell Res. Ther. 7 (2016). https://doi.org/10.1186/s13287-016-0350-z.
C. Lo Sicco, D. Reverberi, C. Balbi, V. Ulivi, E. Principi, L. Pascucci, P. Becherini, M.C. Bosco, L. Varesio, C. Franzin, M. Pozzobon, R. Cancedda, R. Tasso, Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: Endorsement of macrophage polarization, Stem Cells Transl. Med. 6 (2017) 1018–1028. https://doi.org/10.1002/sctm.16-0363.
Liu, L., Jin, X., Hu, C. F., Li, R., Zhou, Z., & Shen, C. X. (2017). Exosomes Derived from Mesenchymal Stem Cells Rescue Myocardial Ischaemia/Reperfusion Injury by Inducing Cardiomyocyte Autophagy Via AMPK and Akt Pathways. Cellular Physiology and Biochemistry, 43, 52–68. https://doi.org/10.1159/000480317
Mao, Q., Lin, C. X., Liang, X. L., Gao, J. S., & Xu, B. (2013). Mesenchymal stem cells overexpressing integrin-linked kinase attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. Molecular Medicine Reports, 7, 1617–1623. https://doi.org/10.3892/mmr.2013.1348
Hatzistergos, K. E., Quevedo, H., Oskouei, B. N., Hu, Q., Feigenbaum, G. S., Margitich, I. S., Mazhari, R., Boyle, A. J., Zambrano, J. P., Rodriguez, J. E., Dulce, R., Pattany, P. M., Valdes, D., Revilla, C., Heldman, A. W., McNiece, I., & Hare, J. M. (2010). Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circulation Research, 107, 913–922. https://doi.org/10.1161/CIRCRESAHA.110.222703
Tan, Y., Nie, W., Chen, C., He, X., Xu, Y., Ma, X., Zhang, J., Tan, M., Rong, P., & Wang, W. (2019). Mesenchymal stem cells alleviate hypoxia-induced oxidative stress and enhance the pro-survival pathways in porcine islets. Experimental Biology and Medicine. https://doi.org/10.1177/1535370219844472
Sun, X. H., Wang, X., Zhang, Y., & Hui, J. (2019). Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486-5p targeting the PTEN/PI3K/AKT signaling pathway. Thrombosis Research, 177, 23–32. https://doi.org/10.1016/j.thromres.2019.02.002
M. Gong, B. Yu, J. Wang, Y. Wang, M. Liu, C. Paul, R.W. Millard, D.S. Xiao, M. Ashraf, M. Xu, Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis, Oncotarget. 8 (2017) 45200–45212. https://doi.org/10.18632/oncotarget.16778.
Liang, X., Zhang, L., Wang, S., Han, Q., & Zhao, R. C. (2016). Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. Journal of Cell Science, 129, 2182–2189. https://doi.org/10.1242/jcs.170373
C. Merino-González, F.A. Zuñiga, C. Escudero, V. Ormazabal, C. Reyes, E. Nova-Lamperti, C. Salomón, C. Aguayo, Mesenchymal stem cell-derived extracellular vesicles promote angiogenesis: Potencial clinical application, Front. Physiol. 7 (2016). https://doi.org/10.3389/fphys.2016.00024.
J. Sheu, F. Lee, C. Yuen, Y. Chen, … T.H.-I.J. of, U. 2015, Combined therapy with shock wave and autologous bone marrow-derived mesenchymal stem cells alleviates left ventricular dysfunction and remodeling through, Elsevier. 193 (2015) 69–83.
Lepidi, S. (2018). Commentary on “Efficient Differentiation of Bone Marrow Mesenchymal Stem Cells into Endothelial Cells in vitro.” European Journal of Vascular and Endovascular Surgery, 55, 266. https://doi.org/10.1016/j.ejvs.2017.11.017
S. Shi, J. Sun, Q. Meng, Y. Yu, H. Huang, T. Ma, Z. Yang, X. Liu, J. Yang, Z. Shen, Sonic hedgehog promotes endothelial differentiation of bone marrow mesenchymal stem cells via VEGF-D, J. Thorac. Dis. 10 (2018) 5476–5488. https://doi.org/10.21037/jtd.2018.09.50.
Lin, P., Correa, D., Kean, T. J., Awadallah, A., Dennis, J. E., & Caplan, A. I. (2014). Serial transplantation and long-term engraftment of intra-arterially delivered clonally derived mesenchymal stem cells to injured bone marrow. Molecular Therapy, 22, 160–168. https://doi.org/10.1038/mt.2013.221
Keating, A. (2012). Mesenchymal stromal cells: New directions. Cell Stem Cell, 10, 709–716. https://doi.org/10.1016/j.stem.2012.05.015
Wang, H. H., Meng, M. B., Wu, Z. Q., Guo, W. H., Jiang, B., Ying, G. G., Zhao, L. J., Yuan, Z. Y., & Wang, P. (2015). Mesenchymal Stem Cells Generate Pericytes to Promote Tumor Recurrence via Vasculogenesis After Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol., 93, E532. https://doi.org/10.1016/j.ijrobp.2015.07.1909
M. Loibl, A. Binder, M. Herrmann, F. Duttenhoefer, R.G. Richards, M. Nerlich, M. Alini, S. Verrier, Direct cell-cell contact between mesenchymal stem cells and endothelial progenitor cells induces a pericyte-like phenotype in vitro, Biomed Res. Int. 2014 (2014). https://doi.org/10.1155/2014/395781.
D. Klein, P. Weißhardt, V. Kleff, H. Jastrow, H.G. Jakob, S. Ergün, Vascular wall-resident CD44+ multipotent stem cells give rise to pericytes and smooth muscle cells and contribute to new vessel maturation, PLoS One. 6 (2011). https://doi.org/10.1371/journal.pone.0020540.
Gökçinar-Yagci, B., Uçkan-Çetinkaya, D., & Çelebi-Saltik, B. (2015). Pericytes: Properties, functions and applications in tissue engineering. Stem Cell Rev. Reports., 11, 549–559. https://doi.org/10.1007/s12015-015-9590-z
Coulson-Thomas, V. J., Coulson-Thomas, Y. M., Gesteira, T. F., & Kao, W. W. Y. (2016). Extrinsic and Intrinsic Mechanisms by Which Mesenchymal Stem Cells Suppress the Immune System. The Ocular Surface, 14, 121–134. https://doi.org/10.1016/j.jtos.2015.11.004
Argani, H. (2019). Anti-HLA antibody: The role of epitopes in organ transplantation. Experimental and Clinical Transplantation, 17, 38–42. https://doi.org/10.6002/ECT.MESOT2018.L41
C.M. Lin, R.G. Gill, Direct and indirect allograft recognition: Pathways dictating graft rejection mechanisms, Curr. Opin. Organ Transplant. 21 (2016). https://doi.org/10.1097/MOT.0000000000000263.
Uccelli, A., & de Rosbo, N. K. (2015). The immunomodulatory function of mesenchymal stem cells: Mode of action and pathways. Annals of the New York Academy of Sciences, 1351, 114–126. https://doi.org/10.1111/nyas.12815
Wu, C., Zhao, Y., Xiao, X., Fan, Y., Kloc, M., Liu, W., Ghobrial, R. M., Lan, P., He, X., & Li, X. C. (2016). Graft-Infiltrating Macrophages Adopt an M2 Phenotype and Are Inhibited by Purinergic Receptor P2X7 Antagonist in Chronic Rejection. American Journal of Transplantation, 16, 2563–2573. https://doi.org/10.1111/ajt.13808
Petersson, E., Östraat, Ö., Ekberg, H., Hansson, J., Simanaitis, M., Brodin, T., Dohlsten, M., & Hedlund, G. (1997). Allogeneic heart transplantation activates alloreactive NK cells. Cellular Immunology, 175, 25–32. https://doi.org/10.1006/cimm.1996.1031
Lu, Y., Liu, J., Liu, Y., Qin, Y., Luo, Q., Wang, Q., & Duan, H. (2015). TLR4 plays a crucial role in MSC-induced inhibition of NK cell function. Biochemical and Biophysical Research Communications, 464, 541–547. https://doi.org/10.1016/j.bbrc.2015.07.002
F. Gao, S.M. Chiu, D.A.L. Motan, Z. Zhang, L. Chen, H.L. Ji, H.F. Tse, Q.L. Fu, Q. Lian, Mesenchymal stem cells and immunomodulation: Current status and future prospects, Cell Death Dis. 7 (2016). https://doi.org/10.1038/cddis.2015.327.
Rashedi, I., Gómez-Aristizábal, A., Wang, X. H., Viswanathan, S., & Keating, A. (2017). TLR3 or TLR4 Activation Enhances Mesenchymal Stromal Cell-Mediated Treg Induction via Notch Signaling. Stem Cells., 35, 265–275. https://doi.org/10.1002/stem.2485
Wang, Y., Chen, X., Cao, W., & Shi, Y. (2014). Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nature Immunology, 15, 1009–1016. https://doi.org/10.1038/ni.3002
Ciavarella, C., & Pasquinelli, G. (2020). The Dual Nature of Mesenchymal Stem Cells (MSCs): Yin and Yang of the Inflammatory Process. Updat. Mesenchymal Induc. Pluripotent Stem Cells. https://doi.org/10.5772/intechopen.82877
Marigo, I., & Dazzi, F. (2011). The immunomodulatory properties of mesenchymal stem cells. Semin. Immunopathol., 33, 593–602. https://doi.org/10.1007/s00281-011-0267-7
Djouad, F., Charbonnier, L.-M., Bouffi, C., Louis-Plence, P., Bony, C., Apparailly, F., Cantos, C., Jorgensen, C., & Noël, D. (2007). Mesenchymal Stem Cells Inhibit the Differentiation of Dendritic Cells Through an Interleukin-6-Dependent Mechanism. Stem Cells., 25, 2025–2032. https://doi.org/10.1634/stemcells.2006-0548
W. hua Liu, J. jin Liu, J. Wu, L. lu Zhang, F. Liu, L. Yin, M. mao Zhang, B. Yu, Novel Mechanism of Inhibition of Dendritic Cells Maturation by Mesenchymal Stem Cells via Interleukin-10 and the JAK1/STAT3 Signaling Pathway, PLoS One. 8 (2013). https://doi.org/10.1371/journal.pone.0055487.
R. Cui, H. Rekasi, M. Hepner-Schefczyk, K. Fessmann, R.M. Petri, K. Bruderek, S. Brandau, M. Jäger, S.B. Flohé, Human mesenchymal stromal/stem cells acquire immunostimulatory capacity upon cross-talk with natural killer cells and might improve the NK cell function of immunocompromised patients, Stem Cell Res. Ther. 7 (2016). https://doi.org/10.1186/s13287-016-0353-9.
Michelo, C. M., Fasse, E., van Cranenbroek, B., Linda, K., van der Meer, A., Abdelrazik, H., & Joosten, I. (2016). Added effects of dexamethasone and mesenchymal stem cells on early Natural Killer cell activation. Transplant Immunology, 37, 1–9. https://doi.org/10.1016/j.trim.2016.04.008
Glass, C. K., & Natoli, G. (2016). Molecular control of activation and priming in macrophages. Nature Immunology, 17, 26–33. https://doi.org/10.1038/ni.3306
R.S. Waterman, S.L. Tomchuck, S.L. Henkle, A.M. Betancourt, A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype, PLoS One. 5 (2010). https://doi.org/10.1371/journal.pone.0010088.
Bernardo, M. E., & Fibbe, W. E. (2013). Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell, 13, 392–402. https://doi.org/10.1016/j.stem.2013.09.006
Abdi, J., Rashedi, I., & Keating, A. (2018). Concise Review: TLR Pathway-miRNA Interplay in Mesenchymal Stromal Cells: Regulatory Roles and Therapeutic Directions. Stem Cells., 36, 1655–1662. https://doi.org/10.1002/stem.2902
Chinnadurai, R., Copland, I. B., Garcia, M. A., Petersen, C. T., Lewis, C. N., Waller, E. K., Kirk, A. D., & Galipeau, J. (2016). Cryopreserved Mesenchymal Stromal Cells Are Susceptible to T-Cell Mediated Apoptosis Which Is Partly Rescued by IFNγ Licensing. Stem Cells., 34, 2429–2442. https://doi.org/10.1002/stem.2415
Kean, T. J., Lin, P., Caplan, A. I., & Dennis, J. E. (2013). MSCs: Delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. https://doi.org/10.1155/2013/732742
Shi, Y., Su, J., Roberts, A. I., Shou, P., Rabson, A. B., & Ren, G. (2012). How mesenchymal stem cells interact with tissue immune responses. Trends in Immunology, 33, 136–143. https://doi.org/10.1016/j.it.2011.11.004
L. Fan, C. Hu, J. Chen, P. Cen, J. Wang, L. Li, Interaction between mesenchymal stem cells and B-cells, Int. J. Mol. Sci. 17 (2016). https://doi.org/10.3390/ijms17050650.
Carmen, G.-C.M., Aitor, C., Vicent, B., Cesar, R.-N., Ana, D., Andrea, S.-P., & Jose, V. (2018). Early reductive stress followed by a late onset oxidative stress in acute myocardial infarction. Free Radical Biology & Medicine, 120, S89. https://doi.org/10.1016/j.freeradbiomed.2018.04.295
G.A. Kurian, R. Rajagopal, S. Vedantham, M. Rajesh, The Role of Oxidative Stress in Myocardial Ischemia and Reperfusion Injury and Remodeling: Revisited, Oxid. Med. Cell. Longev. 2016 (2016). https://doi.org/10.1155/2016/1656450.
Valle-Prieto, A., & Conget, P. A. (2010). Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev., 19, 1885–1893. https://doi.org/10.1089/scd.2010.0093
Liu, T., Ma, X., Ouyang, T., Chen, H., Lin, J., Liu, J., Xiao, Y., Yu, J., & Huang, Y. (2018). SIRT1 reverses senescence via enhancing autophagy and attenuates oxidative stress-induced apoptosis through promoting p53 degradation. International Journal of Biological Macromolecules, 117, 225–234. https://doi.org/10.1016/j.ijbiomac.2018.05.174
Rojas, M., Iyer, S. S., Torres-Gonzalez, E., Neujahr, D. C., Kwon, M., Brigham, K. L., Jones, D. P., & Mora, A. L. (2010). Effect of bone marrow-derived mesenchymal stem cells on endotoxin-induced oxidation of plasma cysteine and glutathione in mice. Stem Cells Int. https://doi.org/10.4061/2010/868076
Mahrouf-Yorgov, M., Augeul, L., Da Silva, C. C., Jourdan, M., Rigolet, M., Manin, S., Ferrera, R., Ovize, M., Henry, A., Guguin, A., Meningaud, J. P., Dubois-Randé, J. L., Motterlini, R., Foresti, R., & Rodriguez, A. M. (2017). Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death and Differentiation, 24, 1224–1238. https://doi.org/10.1038/cdd.2017.51
Desantiago, J., Bare, D. J., & Banach, K. (2013). Ischemia/reperfusion injury protection by mesenchymal stem cell derived antioxidant capacity. Stem Cells Dev., 22, 2497–2507. https://doi.org/10.1089/scd.2013.0136
Sun, C. K., Zhen, Y. Y., Leu, S., Tsai, T. H., Chang, L. T., Sheu, J. J., Chen, Y. L., Chua, S., Chai, H. T., Lu, H. I., Chang, H. W., Lee, F. Y., & Yip, H. K. (2014). Direct implantation versus platelet-rich fibrin-embedded adipose-derived mesenchymal stem cells in treating rat acute myocardial infarction. International Journal of Cardiology, 173, 410–423. https://doi.org/10.1016/j.ijcard.2014.03.015
J. Ni, X. Liu, Y. Yin, P. Zhang, Y.W. Xu, Z. Liu, Exosomes derived from TIMP2-modified human umbilical cord mesenchymal stem cells enhance the repair effect in rat model with myocardial infarction possibly by the Akt/ SFRP2 pathway, Oxid. Med. Cell. Longev. 2019 (2019). https://doi.org/10.1155/2019/1958941.
R.H. ...et.al, Cardiogenic differentiation and transdifferentiation... - Google Scholar, Circ. Res. 103 (2008) 1058–1071.
M. Natsumeda, V. Florea, … A.R.-J. of the, U. 2017, A combination of allogeneic stem cells promotes cardiac regeneration, Onlinejacc.Org. 70 (2017).
Hatzistergos, K. E., Saur, D., Seidler, B., Balkan, W., Breton, M., Valasaki, K., Takeuchi, L. M., Landin, A. M., Khan, A., & Hare, J. M. (2016). Stimulatory Effects of Mesenchymal Stem Cells on cKit+ Cardiac Stem Cells Are Mediated by SDF1/CXCR4 and SCF/cKit Signaling Pathways. Circulation Research, 119, 921–930. https://doi.org/10.1161/CIRCRESAHA.116.309281
Urbanek, K., Rota, M., Cascapera, S., Bearzi, C., Nascimbene, A., De Angelis, A., Hosoda, T., Chimenti, S., Baker, M., Limana, F., Nurzynska, D., Torella, D., Rotatori, F., Rastaldo, R., Musso, E., Quaini, F., Leri, A., Kajstura, J., & Anversa, P. (2005). Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circulation Research, 97, 663–673. https://doi.org/10.1161/01.RES.0000183733.53101.11
O’Neill, H. S., O’Sullivan, J., Porteous, N., Ruiz-Hernandez, E., Kelly, H. M., O’Brien, F. J., & Duffy, G. P. (2018). A collagen cardiac patch incorporating alginate microparticles permits the controlled release of hepatocyte growth factor and insulin-like growth factor-1 to enhance cardiac stem cell migration and proliferation. Journal of Tissue Engineering and Regenerative Medicine, 12, e384–e394. https://doi.org/10.1002/term.2392
Zisa, D., Shabbir, A., Suzuki, G., & Lee, T. (2009). Vascular endothelial growth factor (VEGF) as a key therapeutic trophic factor in bone marrow mesenchymal stem cell-mediated cardiac repair. Biochemical and Biophysical Research Communications, 390, 834–838. https://doi.org/10.1016/j.bbrc.2009.10.058
Tang, J., Wang, J., Kong, X., Yang, J., Guo, L., Zheng, F., Zhang, L., Huang, Y., & Wan, Y. (2009). Vascular endothelial growth factor promotes cardiac stem cell migration via the PI3K/Akt pathway. Experimental Cell Research, 315, 3521–3531. https://doi.org/10.1016/j.yexcr.2009.09.026
Haider, H. K., Jiang, S., Idris, N. M., & Ashraf, M. (2008). IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1α/CXCR4 signaling to promote myocardial repair. Circulation Research, 103, 1300–1308. https://doi.org/10.1161/CIRCRESAHA.108.186742
Park, S., Jang, H., Kim, B. S., Hwang, C., Jeong, G. S., & Park, Y. (2017). Directional migration of mesenchymal stem cells under an SDF-1α gradient on a microfluidic device. PLoS ONE. https://doi.org/10.1371/journal.pone.0184595
Chen, M. F., Lee, B. C., Hsu, H. C., Tseng, W. Y. I., Chen, C. Y., Lin, H. J., Ho, Y. L., & Su, M. J. (2009). Cell therapy generates a favourable chemokine gradient for stem cell recruitment into the infarcted heart in rabbits. European Journal of Heart Failure, 11, 238–245. https://doi.org/10.1093/eurjhf/hfn035
K. Zuo, D. Kuang, Y. Wang, Y. Xia, W. Tong, X. Wang, Y. Chen, Y. Duan, G. Wang, SCF/c-kit transactivates CXCR4-serine 339 phosphorylation through G protein-coupled receptor kinase 6 and regulates cardiac stem cell migration, Sci. Rep. 6 (2016). https://doi.org/10.1038/srep26812.
Leri, A., Rota, M., Hosoda, T., Goichberg, P., & Anversa, P. (2014). Cardiac stem cell niches. Stem Cell Res., 13, 631–646. https://doi.org/10.1016/j.scr.2014.09.001
Gnecchi, M., He, H., Noiseux, N., Liang, O. D., Zhang, L., Morello, F., Mu, H., Melo, L. G., Pratt, R. E., Ingwall, J. S., & Dzau, V. J. (2006). Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. The FASEB Journal, 20, 661–669. https://doi.org/10.1096/fj.05-5211com
Mirotsou, M., Zhang, Z., Deb, A., Zhang, L., Gnecchi, M., Noiseux, N., Mu, H., Pachori, A., & Dzau, V. (2007). Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc. Natl. Acad. Sci. U. S. A., 104, 1643–1648. https://doi.org/10.1073/pnas.0610024104
J. Ma, Y. Zhao, L. Sun, X. Sun, … X.Z.-S. cells, U. 2017, Exosomes derived from AKt‐modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet, Wiley Online Libr. (2016).
Wang, X., Zhao, T., Huang, W., Wang, T., Qian, J., Xu, M., Kranias, E. G., Wang, Y., & Fan, G. C. (2009). Hsp20-engineered mesenchymal stem cells are resistant to oxidative stress via enhanced activation of Akt and increased secretion of growth factors. Stem Cells., 27, 3021–3031. https://doi.org/10.1002/stem.230
Tang, J. M., Wang, J. N., Zhang, L., Zheng, F., Yang, J. Y., Kong, X., Guo, L. Y., Chen, L., Huang, Y. Z., Wan, Y., & Chen, S. Y. (2011). VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovascular Research, 91, 402–411. https://doi.org/10.1093/cvr/cvr053
J. Bobi, N. Solanes, R. Fernández-Jiménez, C. Galán-Arriola, A.P. Dantas, L. Fernández-Friera, C. Gálvez-Montón, E. Rigol-Monzó, J. Agüero, J. Ramírez, M. Roqué, A. Bayés-Genís, J. Sánchez-González, A. García-Álvarez, M. Sabaté, S. Roura, B. Ibáñez, M. Rigol, Intracoronary administration of allogeneic adipose tissue-derived mesenchymal stem cells improves myocardial perfusion but not left ventricle function, in a translational model of acute myocardial infarction, J. Am. Heart Assoc. 6 (2017). https://doi.org/10.1161/JAHA.117.005771.
Huang, L., Yang, L., Ding, Y., Jiang, X., Xia, Z., & You, Z. (2020). Human umbilical cord mesenchymal stem cells-derived exosomes transfers microRNA-19a to protect cardiomyocytes from acute myocardial infarction by targeting SOX6. Cell Cycle, 19, 339–353. https://doi.org/10.1080/15384101.2019.1711305
Chen, R., Cai, X., Liu, J., Bai, B., & Li, X. (2018). Sphingosine 1-phosphate promotes mesenchymal stem cell-mediated cardioprotection against myocardial infarction via ERK1/2-MMP-9 and Akt signaling axis. Life Sciences, 215, 31–42. https://doi.org/10.1016/j.lfs.2018.10.047
Ceccariglia, S., Cargnoni, A., Silini, A. R., & Parolini, O. (2020). Autophagy: A potential key contributor to the therapeutic action of mesenchymal stem cells. Autophagy, 16, 28–37. https://doi.org/10.1080/15548627.2019.1630223
Z. Zhang, C. Yang, M. Shen, M. Yang, Z. Jin, L. Ding, W. Jiang, J. Yang, H. Chen, F. Cao, T. Hu, Autophagy mediates the beneficial effect of hypoxic preconditioning on bone marrow mesenchymal stem cells for the therapy of myocardial infarction, Stem Cell Res. Ther. 8 (2017). https://doi.org/10.1186/s13287-017-0543-0.
O. Ham, S.Y. Lee, C.Y. Lee, J.H. Park, J. Lee, H.H. Seo, M.J. Cha, E. Choi, S. Kim, K.C. Hwang, Let-7b suppresses apoptosis and autophagy of human mesenchymal stem cells transplanted into ischemia/reperfusion injured heart 7by targeting caspase-3, Stem Cell Res. Ther. 6 (2015). https://doi.org/10.1186/s13287-015-0134-x.
Li, T., Gu, J., Yang, O., Wang, J., Wang, Y., & Kong, J. (2020). Bone Marrow Mesenchymal Stem Cell-Derived Exosomal miRNA-29c Decreases Cardiac Ischemia/Reperfusion Injury Through Inhibition of Excessive Autophagy via the PTEN/Akt/mTOR Signaling Pathway. Circulation Journal, 84, 1304–1311. https://doi.org/10.1253/circj.CJ-19-1060
Horn, M. A., & Trafford, A. W. (2016). Aging and the cardiac collagen matrix: Novel mediators of fibrotic remodelling. Journal of Molecular and Cellular Cardiology, 93, 175–185. https://doi.org/10.1016/j.yjmcc.2015.11.005
Li, L., Zhang, S., Zhang, Y., Yu, B., Xu, Y., & Guan, Z. (2009). Paracrine action mediate the antifibrotic effect of transplanted mesenchymal stem cells in a rat model of global heart failure. Molecular Biology Reports, 36, 725–731. https://doi.org/10.1007/s11033-008-9235-2
Chen, Y. L., Sun, C. K., Tsai, T. H., Chang, L. T., Leu, S., Zhen, Y. Y., Sheu, J. J., Chua, S., Yeh, K. H., Lu, H. I., Chang, H. W., Lee, F. Y., & Yip, H. K. (2015). Adipose-derived mesenchymal stem cells embedded in platelet-rich fibrin scaffolds promote angiogenesis, preserve heart function, and reduce left ventricular remodeling in rat acute myocardial infarction. Am. J. Transl. Res., 7, 781–803.
Kandalam, V., Basu, R., Abraham, T., Wang, X., Soloway, P. D., Jaworski, D. M., Oudit, G. Y., & Kassiri, Z. (2010). TIMP2 deficiency accelerates adverse post-myocardial infarction remodeling because of enhanced MT1-MMP activity despite lack of MMP2 activation. Circulation Research, 106, 796–808. https://doi.org/10.1161/CIRCRESAHA.109.209189
Li, S. H., Guo, J., Wu, J., Sun, Z., Han, M., Shan, S. W., Deng, Z., Yang, B. B., Weisel, R. D., & Li, R. K. (2013). miR-17 targets tissue inhibitor of metalloproteinase 1 and 2 to modulate cardiac matrix remodeling. The FASEB Journal, 27, 4254–4265. https://doi.org/10.1096/fj.13-231688
Kim, S. W., Lee, D. W., Yu, L. H., Zhang, H. Z., Kim, C. E., Kim, J. M., Park, T. H., Cha, K. S., Seo, S. Y., Roh, M. S., Lee, K. C., Jung, J. S., & Kim, M. H. (2012). Mesenchymal stem cells overexpressing GCP-2 improve heart function through enhanced angiogenic properties in a myocardial infarction model. Cardiovascular Research, 95, 495–506. https://doi.org/10.1093/cvr/cvs224
J. Liu, P. Zhu, P. Song, W. Xiong, H. Chen, W. Peng, S. Wang, S. Li, Z. Fu, Y. Wang, H. Wang, Pretreatment of Adipose Derived Stem Cells with Curcumin Facilitates Myocardial Recovery via Antiapoptosis and Angiogenesis, Stem Cells Int. 2015 (2015). https://doi.org/10.1155/2015/638153.
Teng, X., Chen, L., Chen, W., Yang, J., Yang, Z., & Shen, Z. (2015). Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cellular Physiology and Biochemistry, 37, 2415–2424. https://doi.org/10.1159/000438594
Fang, J., Chen, L., Fan, L., Wu, L., Chen, X., Li, W., Lin, Y., & Wang, W. (2011). Enhanced therapeutic effects of mesenchymal stem cells on myocardial infarction by ischemic postconditioning through paracrine mechanisms in rats. Journal of Molecular and Cellular Cardiology, 51, 839–847. https://doi.org/10.1016/j.yjmcc.2011.06.013
Chi, N. H., Yang, M. C., Chung, T. W., Chen, J. Y., Chou, N. K., & Wang, S. S. (2012). Cardiac repair achieved by bone marrow mesenchymal stem cells/silk fibroin/hyaluronic acid patches in a rat of myocardial infarction model. Biomaterials, 33, 5541–5551. https://doi.org/10.1016/j.biomaterials.2012.04.030
K. Wu, B. Zhou, C. Yu, B. Cui, S. Lu, … Z.H.-T.A. of thoracic, U. 2007, Therapeutic potential of human umbilical cord derived stem cells in a rat myocardial infarction model, Elsevier. 83 (2007) 1491–1498.
Zhao, L., Liu, X., Zhang, Y., Liang, X., Ding, Y., Xu, Y., Fang, Z., & Zhang, F. (2016). Enhanced cell survival and paracrine effects of mesenchymal stem cells overexpressing hepatocyte growth factor promote cardioprotection in myocardial infarction. Experimental Cell Research, 344, 30–39. https://doi.org/10.1016/j.yexcr.2016.03.024
S. Deng, X. Zhou, Z. Ge, Y. Song, H. Wang, X. Liu, D. Zhang, Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization, Int. J. Biochem. Cell Biol. 114 (2019). https://doi.org/10.1016/j.biocel.2019.105564.
Dayan, V., Yannarelli, G., Billia, F., Filomeno, P., Wang, X. H., Davies, J. E., & Keating, A. (2011). Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Research in Cardiology, 106, 1299–1310. https://doi.org/10.1007/s00395-011-0221-9
Xu, R., Zhang, F., Chai, R., Zhou, W., Hu, M., Liu, B., Chen, X., Liu, M., Xu, Q., Liu, N., & Liu, S. (2019). Exosomes derived from pro-inflammatory bone marrow-derived mesenchymal stem cells reduce inflammation and myocardial injury via mediating macrophage polarization. Journal of Cellular and Molecular Medicine, 23, 7617–7631. https://doi.org/10.1111/jcmm.14635
Zhao, J., Li, X., Hu, J., Chen, F., Qiao, S., Sun, X., Gao, L., Xie, J., & Xu, B. (2019). Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovascular Research, 115, 1205–1216. https://doi.org/10.1093/cvr/cvz040
Han, D., Huang, W., Li, X., Gao, L., Su, T., Li, X., Ma, S., Liu, T., Li, C., Chen, J., Gao, E., & Cao, F. (2016). Melatonin facilitates adipose-derived mesenchymal stem cells to repair the murine infarcted heart via the SIRT1 signaling pathway. Journal of Pineal Research, 60, 178–192. https://doi.org/10.1111/jpi.12299
Li, N., Yang, Y. J., Qian, H. Y., Li, Q., Zhang, Q., Li, X. D., Dong, Q. T., Xu, H., Song, L., & Zhang, H. (2015). Intravenous administration of atorvastatin-pretreated mesenchymal stem cells improves cardiac performance after acute myocardial infarction: Role of CXCR4. Am. J. Transl. Res., 7, 1058–1070.
Zeng, B., Chen, H., Zhu, C., Ren, X., Lin, G., & Cao, F. (2008). Effects of combined mesenchymal stem cells and heme oxygenase-1 therapy on cardiac performance. Eur. J. Cardio-Thoracic Surg., 34, 850–856. https://doi.org/10.1016/j.ejcts.2008.05.049
Paul, A., Srivastava, S., Chen, G., Shum-Tim, D., & Prakash, S. (2013). Functional Assessment of Adipose Stem Cells for Xenotransplantation Using Myocardial Infarction Immunocompetent Models: Comparison with Bone Marrow Stem Cells. Cell Biochemistry and Biophysics, 67, 263–273. https://doi.org/10.1007/s12013-011-9323-0
Henning, R. J., Burgos, J. D., Ondrovic, L., Sanberg, P., Balis, J., & Morgan, M. B. (2006). Human umbilical cord blood progenitor cells are attracted to infarcted myocardium and significantly reduce myocardial infarction size. Cell Transplantation, 15, 647–658. https://doi.org/10.3727/000000006783981611
Mias, C., Lairez, O., Trouche, E., Roncalli, J., Calise, D., Seguelas, M. H., Ordener, C., Piercecchi-Marti, M. D., Auge, N., Salvayre, A. N., Bourin, P., Parini, A., & Cussac, D. (2009). Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells., 27, 2734–2743. https://doi.org/10.1002/stem.169
Y. Zhao, X. Sun, W. Cao, J. Ma, L. Sun, H. Qian, W. Zhu, W. Xu, Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Relieve Acute Myocardial Ischemic Injury, Stem Cells Int. 2015 (2015). https://doi.org/10.1155/2015/761643.
Yao, L. T., Zhao, Q., Qin, X., Shen, L., Cheng, L., Ge, J., & Phillips, M. I. (2005). Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Annals of Thoracic Surgery, 80, 229–237. https://doi.org/10.1016/j.athoracsur.2005.02.072
Cho, J., Zhai, P., Maejima, Y., & Sadoshima, J. (2011). Myocardial injection with GSK-3β-overexpressing bone marrow-derived mesenchymal stem cells attenuates cardiac dysfunction after myocardial infarction. Circulation Research, 108, 478–489. https://doi.org/10.1161/CIRCRESAHA.110.229658
Zhang, W., Liu, X. C., Yang, L., Zhu, D. L., Zhang, Y. D., Chen, Y., & Zhang, H. Y. (2013). Wharton’s jelly-derived mesenchymal stem cells promote myocardial regeneration and cardiac repair after miniswine acute myocardial infarction. Coronary Artery Disease, 24, 549–558. https://doi.org/10.1097/MCA.0b013e3283640f00
Jang, J., Park, H. J., Kim, S. W., Kim, H., Park, J. Y., Na, S. J., Kim, H. J., Park, M. N., Choi, S. H., Park, S. H., Kim, S. W., Kwon, S. M., Kim, P. J., & Cho, D. W. (2017). 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials. https://doi.org/10.1016/j.biomaterials.2016.10.026
Kim, H., Bae, C., Kook, Y. M., Koh, W. G., Lee, K., & Park, M. H. (2019). Mesenchymal stem cell 3D encapsulation technologies for biomimetic microenvironment in tissue regeneration. Stem Cell Research & Therapy. https://doi.org/10.1186/s13287-018-1130-8
T.A. Et.al, Contact guidance for cardiac tissue engineering using 3D bioprinted gelatin patterned hydrogel, Biofabrication. 10 (2018).
Melhem, M. R., Park, J., Knapp, L., Reinkensmeyer, L., Cvetkovic, C., Flewellyn, J., Lee, M. K., Jensen, T. W., Bashir, R., Kong, H., & Schook, L. B. (2017). 3D Printed Stem-Cell-Laden, Microchanneled Hydrogel Patch for the Enhanced Release of Cell-Secreting Factors and Treatment of Myocardial Infarctions. ACS Biomaterials Science & Engineering. https://doi.org/10.1021/acsbiomaterials.6b00176
Rahmi, G., Pidial, L., Silva, A. K. A., Blondiaux, E., Meresse, B., Gazeau, F., Autret, G., Balvay, D., Cuenod, C. A., Perretta, S., Tavitian, B., Wilhelm, C., Cellier, C., & Clément, O. (2016). Designing 3D mesenchymal stem cell sheets merging magnetic and fluorescent features: When cell sheet technology meets image-guided cell therapy. Theranostics., 6, 739–751. https://doi.org/10.7150/thno.14064
Park, S. J., Kim, R. Y., Park, B. W., Lee, S., Choi, S. W., Park, J. H., Choi, J. J., Kim, S. W., Jang, J., Cho, D. W., Chung, H. M., Moon, S. H., Ban, K., & Park, H. J. (2019). Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nature Communications. https://doi.org/10.1038/s41467-019-11091-2
Mombini, S., Mohammadnejad, J., Bakhshandeh, B., Narmani, A., Nourmohammadi, J., Vahdat, S., & Zirak, S. (2019). Chitosan-PVA-CNT nanofibers as electrically conductive scaffolds for cardiovascular tissue engineering. International Journal of Biological Macromolecules. https://doi.org/10.1016/j.ijbiomac.2019.08.046
Sokolowska, P., Zukowski, K., Lasocka, I., Szulc-Dabrowska, L., & Jastrzebska, E. (2020). Human mesenchymal stem cell (hMSC) differentiation towards cardiac cells using a new microbioanalytical method. The Analyst, 145, 3017–3028. https://doi.org/10.1039/c9an02366f
S. Pérez-Rodríguez, E. Tomás-González, J.M. García-Aznar, 3D cell migration studies for chemotaxis on microfluidic-based chips: A comparison between cardiac and dermal fibroblasts, Bioengineering. 5 (2018). https://doi.org/10.3390/bioengineering5020045.
Chikarmane, V., & Peterson, C. (2008). A computational model for understanding stem cell, trophectoderm and endoderm lineage determination. PLoS ONE. https://doi.org/10.1371/journal.pone.0003478
Garikipati, V. N. S., Shoja-Taheri, F., Davis, M. E., & Kishore, R. (2018). Extracellular vesicles and the application of system biology and computational modeling in cardiac repair. Circulation Research. https://doi.org/10.1161/CIRCRESAHA.117.311215
Kernik, D. C., Morotti, S., Di Wu, H., Garg, P., Duff, H. J., Kurokawa, J., Jalife, J., Wu, J. C., Grandi, E., & Clancy, C. E. (2019). A computational model of induced pluripotent stem-cell derived cardiomyocytes incorporating experimental variability from multiple data sources. Journal of Physiology, 597, 4533–4564. https://doi.org/10.1113/JP277724
Mayourian, J., Cashman, T. J., Ceholski, D. K., Johnson, B. V., Sachs, D., Kaji, D. A., Sahoo, S., Hare, J. M., Hajjar, R. J., Sobie, E. A., & Costa, K. D. (2017). Experimental and Computational Insight into Human Mesenchymal Stem Cell Paracrine Signaling and Heterocellular Coupling Effects on Cardiac Contractility and Arrhythmogenicity. Circulation Research. https://doi.org/10.1161/CIRCRESAHA.117.310796
Consolo, F., Bariani, C., Mantalaris, A., Montevecchi, F., Redaelli, A., & Morbiducci, U. (2012). Computational modeling for the optimization of a cardiogenic 3D bioprocess of encapsulated embryonic stem cells. Biomechanics and Modeling in Mechanobiology. https://doi.org/10.1007/s10237-011-0308-0
Roberts, E. G., Piekarski, B. L., Huang, K., Emani, S., Wong, J. Y., & Emani, S. M. (2019). Evaluation of Placental Mesenchymal Stem Cell Sheets for Myocardial Repair and Regeneration. Tissue Eng. - Part A. https://doi.org/10.1089/ten.tea.2018.0035
Tanaka, Y., Shirasawa, B., Takeuchi, Y., Kawamura, D., Nakamura, T., Samura, M., Nishimoto, A., Ueno, K., Morikage, N., Hosoyama, T., & Hamano, K. (2016). Autologous preconditioned mesenchymal stem cell sheets improve left ventricular function in a rabbit old myocardial infarction model. Am. J. Transl. Res., 8, 2222–2233.
M. Miklíková, D. Jarkovská, M. Čedíková, J. Švíglerová, J. Kuncová, L. Nalos, T. Kubíková, V. Liška, M. Holubová, D. Lysák, M. Králíčková, L. Vištejnová, M. Štengl, Beneficial effects of mesenchymal stem cells on adult porcine cardiomyocytes in non-contact co-culture, Physiol. Res. 67 (2018) S619–S631. https://doi.org/10.33549/physiolres.934051.
Aguirre, A., Planell, J. A., & Engel, E. (2010). Dynamics of bone marrow-derived endothelial progenitor cell/mesenchymal stem cell interaction in co-culture and its implications in angiogenesis. Biochemical and Biophysical Research Communications, 400, 284–291. https://doi.org/10.1016/j.bbrc.2010.08.073
Heo, D. N., Hospodiuk, M., & Ozbolat, I. T. (2019). Synergistic interplay between human MSCs and HUVECs in 3D spheroids laden in collagen/fibrin hydrogels for bone tissue engineering. Acta Biomaterialia. https://doi.org/10.1016/j.actbio.2019.02.046
Lemcke, H., Gaebel, R., Skorska, A., Voronina, N., Lux, C. A., Petters, J., Sasse, S., Zarniko, N., Steinhoff, G., & David, R. (2017). Mechanisms of stem cell based cardiac repair-gap junctional signaling promotes the cardiac lineage specification of mesenchymal stem cells. Science and Reports. https://doi.org/10.1038/s41598-017-10122-6
Stone, L. L. H., Chappuis, E., Marquez, M., McFalls, E. O., Kelly, R. F., & Butterick, T. (2019). Mitochondrial Respiratory Capacity is Restored in Hibernating Cardiomyocytes Following Co-Culture with Mesenchymal Stem Cells. Cell Med., 11, 215517901983493. https://doi.org/10.1177/2155179019834938
M.H. Norahan, M. Pourmokhtari, M.R. Saeb, B. Bakhshi, M. Soufi Zomorrod, N. Baheiraei, Electroactive cardiac patch containing reduced graphene oxide with potential antibacterial properties, Mater. Sci. Eng. C. (2019). https://doi.org/10.1016/j.msec.2019.109921.
K. Roshanbinfar, Z. Mohammadi, A. Sheikh-Mahdi Mesgar, M.M. Dehghan, O.P. Oommen, J. Hilborn, F.B. Engel, Carbon nanotube doped pericardial matrix derived electroconductive biohybrid hydrogel for cardiac tissue engineering, Biomater. Sci. (2019). https://doi.org/10.1039/c9bm00434c.
Dong, Y., Hong, M., Dai, R., Wu, H., & Zhu, P. (2020). Engineered bioactive nanoparticles incorporated biofunctionalized ECM/silk proteins based cardiac patches combined with MSCs for the repair of myocardial infarction: In vitro and in vivo evaluations. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2019.135976
Shojaie, S., Rostamian, M., Samadi, A., Alvani, M. A. S., Khonakdar, H. A., Goodarzi, V., Zarrintaj, R., Servatan, M., Asefnejad, A., Baheiraei, N., & Saeb, M. R. (2019). Electrospun electroactive nanofibers of gelatin-oligoaniline/Poly (vinyl alcohol) templates for architecting of cardiac tissue with on-demand drug release. Polymers for Advanced Technologies, 30, 1473–1483. https://doi.org/10.1002/pat.4579
Musiał-Wysocka, A., Kot, M., & Majka, M. (2019). The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplantation, 28, 801–812. https://doi.org/10.1177/0963689719837897
A. Bongso, C.-Y. Fong, The Therapeutic Potential, Challenges and Future Clinical Directions of Stem Cells from the Wharton’s Jelly of the Human Umbilical Cord, Stem Cell Rev. Reports 2012 92. 9 (2012) 226–240. https://doi.org/10.1007/S12015-012-9418-Z.
I.R. Murray, C.C. West, W.R. Hardy, A.W. James, T.S. Park, A. Nguyen, T. Tawonsawatruk, L. Lazzari, C. Soo, B. Péault, Natural history of mesenchymal stem cells, from vessel walls to culture vessels, Cell. Mol. Life Sci. 2013 718. 71 (2013) 1353–1374. https://doi.org/10.1007/S00018-013-1462-6.
K. S, E. H, S. J, K. H, B. K, Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue, Stem Cells. 24 (2006) 1294–1301. https://doi.org/10.1634/STEMCELLS.2005-0342.
van der S. TI, J. of L. SJ, A. P, van B. E, G. M, S. JP, C. MJ, D. PA, C. SA, Human relevance of pre-clinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease, Cardiovasc. Res. 91 (2011) 649–658. https://doi.org/10.1093/CVR/CVR113.
Majka, M., Sułkowski, M., Badyra, B., & Musiałek, P. (2017). Concise Review: Mesenchymal Stem Cells in Cardiovascular Regeneration: Emerging Research Directions and Clinical Applications. Stem Cells Translational Medicine, 6, 1859–1867. https://doi.org/10.1002/sctm.16-0484
Z. Chen, L. Chen, C. Zeng, W.E. Wang, Functionally improved mesenchymal stem cells to better treat myocardial infarction, Stem Cells Int. 2018 (2018). https://doi.org/10.1155/2018/7045245.
Squillaro, T., Peluso, G., & Galderisi, U. (2016). Clinical trials with mesenchymal stem cells: An update. Cell Transplantation, 25, 829–848. https://doi.org/10.3727/096368915X689622
Lee, J. W., Lee, S. H., Youn, Y. J., Ahn, M. S., Kim, J. Y., Yoo, B. S., Yoon, J., Kwon, W., Hong, I. S., Lee, K., Kwan, J., Park, K. S., Choi, D., Jang, Y. S., & Hong, M. K. (2014). A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. Journal of Korean Medical Science, 29, 23–31. https://doi.org/10.3346/jkms.2014.29.1.23
Hare, J. M., Traverse, J. H., Henry, T. D., Dib, N., Strumpf, R. K., Schulman, S. P., Gerstenblith, G., DeMaria, A. N., Denktas, A. E., Gammon, R. S., Hermiller, J. B., Reisman, M. A., Schaer, G. L., Sherman, W., & Randomized, A. (2009). Double-Blind, Placebo-Controlled, Dose-Escalation Study of Intravenous Adult Human Mesenchymal Stem Cells (Prochymal) After Acute Myocardial Infarction. Journal of the American College of Cardiology, 54, 2277–2286. https://doi.org/10.1016/j.jacc.2009.06.055
L.R. Gao, Y. Chen, N.K. Zhang, X.L. Yang, H.L. Liu, Z.G. Wang, X.Y. Yan, Y. Wang, Z.M. Zhu, T.C. Li, L.H. Wang, H.Y. Chen, Y.D. Chen, C.L. Huang, P. Qu, C. Yao, B. Wang, G.H. Chen, Z.M. Wang, Z.Y. Xu, J. Bai, D. Lu, Y.H. Shen, F. Guo, M.Y. Liu, Y. Yang, Y.C. Ding, Y. Yang, H.T. Tian, Q.A. Ding, L.N. Li, X.C. Yang, X. Hu, Intracoronary infusion of Wharton’s jelly-derived mesenchymal stem cells in acute myocardial infarction: Double-blind, randomized controlled trial, BMC Med. 13 (2015). https://doi.org/10.1186/s12916-015-0399-z.
A. Chullikana, A. Sen Majumdar, S. Gottipamula, S. Krishnamurthy, A.S. Kumar, V.S. Prakash, P.K. Gupta, Randomized, double-blind, phase I/II study of intravenous allogeneic mesenchymal stromal cells in acute myocardial infarction, Cytotherapy. 17 (2015) 250–261. https://doi.org/10.1016/j.jcyt.2014.10.009.
F. V., R. A.C., D. D.L., E.-K. J., N. M., B. M.N., T. B.A., K. A., S. I.H., L. A.M., M. M., G. S., L. M.H., B. J.J., H. R.C., C. M.G., V. K., P. M.V., G. E., M. R., D. C., A. F., V.-C. M., S. R.G., D. D., C. L.V., R. K.N., M. A., H. A.W., M. R.D., H. J.M., Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (The TRIDENT study), Circ. Res. 121 (2017) 1279–1290. https://doi.org/10.1161/CIRCRESAHA.117.311827 LK - http://findit.library.jhu.edu/resolve?sid=EMBASE&issn=15244571&id=doi:10.1161%2FCIRCRESAHA.117.311827&atitle=Dose+comparison+study+of+allogeneic+mesenchymal+stem+cells+in+patients+with+ischemic+cardiomyopathy+%28The+TRIDENT+study%29&stitle=Circ.+Res.&title=Circulation+Research&volume=121&issue=11&spage=1279&epage=1290&aulast=Florea&aufirst=Victoria&auinit=V.&aufull=Florea+V.&coden=CIRUA&isbn=&pages=1279-1290&date=2017&auinit1=V&auinitm=.
J.M. Hare, J.E. Fishman, G. Gerstenblith, D.L. DiFede Velazquez, J.P. Zambrano, V.Y. Suncion, M. Tracy, E. Ghersin, P. V. Johnston, J.A. Brinker, E. Breton, J. Davis-Sproul, I.H. Schulman, J. Byrnes, A.M. Mendizabal, M.H. Lowery, D. Rouy, P. Altman, C. Wong Po Foo, P. Ruiz, A. Amador, J. Da Silva, I.K. McNiece, A.W. Heldman, Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial, JAMA - J. Am. Med. Assoc. 308 (2012) 2369–2379. https://doi.org/10.1001/jama.2012.25321.
Heldman, A. W., DiFede, D. L., Fishman, J. E., Zambrano, J. P., Trachtenberg, B. H., Karantalis, V., Mushtaq, M., Williams, A. R., Suncion, V. Y., McNiece, I. K., Ghersin, E., Soto, V., Lopera, G., Miki, R., Willens, H., Hendel, R., Mitrani, R., Pattany, P., Feigenbaum, G., … Hare, J. M. (2014). Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The TAC-HFT randomized trial. JAMA - J. Am. Med. Assoc., 311, 62–73. https://doi.org/10.1001/jama.2013.282909
Guijarro, D., Lebrin, M., Lairez, O., Bourin, P., Piriou, N., Pozzo, J.-L., Lande, G., Berry, M., Le Tourneau, T., Cussac, D., Sensebe, L., Gross, F., Lamirault, G., Huynh, A., Manrique, A., Ruidavet, J. B., Elbaz, M., Trochu, J. N., Parini, A., … Roncalli, J. (2016). Intramyocardial transplantation of mesenchymal stromal cells for chronic myocardial ischemia and impaired left ventricular function: Results of the MESAMI 1 pilot trial. International Journal of Cardiology, 209, 258–265. https://doi.org/10.1016/j.ijcard.2016.02.016
Mathiasen, A. B., Haack-Sørensen, M., Jørgensen, E., & Kastrup, J. (2013). Autotransplantation of mesenchymal stromal cells from bone-marrow to heart in patients with severe stable coronary artery disease and refractory angina - Final 3-year follow-up. International Journal of Cardiology, 170, 246–251. https://doi.org/10.1016/j.ijcard.2013.10.079
A.A. Qayyum, A.B. Mathiasen, S. Helqvist, E. Jørgensen, M. Haack-Sørensen, A. Ekblond, J. Kastrup, Autologous adipose-derived stromal cell treatment for patients with refractory angina (MyStromalCell Trial): 3-years follow-up results, J. Transl. Med. 17 (2019). https://doi.org/10.1186/s12967-019-2110-1.
Bartolucci, J., Verdugo, F. J., González, P. L., Larrea, R. E., Abarzua, E., Goset, C., Rojo, P., Palma, I., Lamich, R., Pedreros, P. A., Valdivia, G., Lopez, V. M., Nazzal, C., Alcayaga-Miranda, F., Cuenca, J., Brobeck, M. J., Patel, A. N., Figueroa, F. E., & Khoury, M. (2017). Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: A phase 1/2 randomized controlled trial (RIMECARD trial [Randomized clinical trial of intravenous infusion umbilical cord mesenchymal. Circulation Research, 121, 1192–1204. https://doi.org/10.1161/CIRCRESAHA.117.310712
Butler, J., Epstein, S. E., Greene, S. J., Quyyumi, A. A., Sikora, S., Kim, R. J., Anderson, A. S., Wilcox, J. E., Tankovich, N. I., Lipinski, M. J., Ko, Y. A., Margulies, K. B., Cole, R. T., Skopicki, H. A., & Gheorghiade, M. (2017). Intravenous Allogeneic Mesenchymal Stem Cells for Nonischemic Cardiomyopathy: Safety and Efficacy Results of a Phase II-A Randomized Trial. Circulation Research, 120, 332–340. https://doi.org/10.1161/CIRCRESAHA.116.309717
Mathiasen, A. B., Qayyum, A. A., Jørgensen, E., Helqvist, S., Kofoed, K. F., Haack-Sørensen, M., Ekblond, A., & Kastrup, J. (2020). Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: Final 4-year follow-up of the MSC-HF trial. European Journal of Heart Failure, 22, 884–892. https://doi.org/10.1002/ejhf.1700
Vrtovec, B., Poglajen, G., Sever, M., Lezaic, L., Domanovic, D., Cernelc, P., Haddad, F., & Torre-Amione, G. (2011). Effects of intracoronary stem cell transplantation in patients with dilated cardiomyopathy. Journal of Cardiac Failure, 17, 272–281. https://doi.org/10.1016/j.cardfail.2010.11.007
Houtgraaf, J. H., Den Dekker, W. K., Van Dalen, B. M., Springeling, T., De Jong, R., Van Geuns, R. J., Geleijnse, M. L., Fernandez-Aviles, F., Zijlsta, F., Serruys, P. W., & Duckers, H. J. (2012). First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. Journal of the American College of Cardiology, 59, 539–540. https://doi.org/10.1016/j.jacc.2011.09.065
Acknowledgements
SG would like to acknowledge Indian Council of Medical Research (ICMR) for fellowship (3/1/3/JRF-2015/HRD-LS/90/40282/91). AS would like to acknowledge Indian Institute of Technology (IIT) Madras for HTRA fellowship. The authors would also like to acknowledge the support by Department of Biotechnology (DBT), India for their funding and support (BT/PR8587/MED/31/236/2013).
Author information
Authors and Affiliations
Contributions
Conceptualization: [Santosh Gupta], [Akriti Sharma], [Rama Shanker Verma]; Methodology: [Akriti Sharma], [Santosh Gupta], [Rama Shanker Verma]; Formal analysis and investigation: [Santosh Gupta], [Akriti Sharma]; Writing—original draft preparation: [Akriti Sharma], [Santosh Gupta], [Archana S], [Rama; Writing—review and editing: [Santosh Gupta], [Akriti Sharma], [Archana S], [Rama Shanker Verma]; Funding acquisition: [Not Applicable]; Resources: [Not Applicable]; Supervision: [Rama Shanker Verma].
[Santosh Gupta] and [Akriti Sharma] has contributed equally as the first author. Their names can be interchangeably used in the first position.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics Approval
Not Applicable.
Consent to Participate
Not Applicable.
Consent to Publish
Not Applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, A., Gupta, S., Archana, S. et al. Emerging Trends in Mesenchymal Stem Cells Applications for Cardiac Regenerative Therapy: Current Status and Advances. Stem Cell Rev and Rep 18, 1546–1602 (2022). https://doi.org/10.1007/s12015-021-10314-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-021-10314-8