Abstract
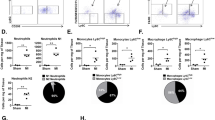
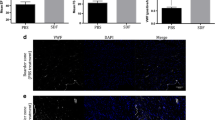
Given the established anti-inflammatory properties of mesenchymal stromal cells (MSCs), we investigated their effect on inflammatory cell infiltration of ischemic cardiac tissue and cardiac function. We employed two types of MSCs, human bone marrow-derived (BM) MSCs and human umbilical cord perivascular cells in an experimental acute myocardial infarction (MI) model with the immune-deficient NOD/SCID gamma null mouse. Cells were infused 48 h after induction of MI and mice assessed 24 h later (72 h after MI) for bone marrow (BM), circulating and cardiac tissue-infiltrating monocytes/macrophages. We showed that in the presence of either MSC type, overall macrophage/monocyte levels were reduced, including pro-inflammatory M1-type macrophages, while the proportion of alternatively activated M2-type macrophages was significantly increased in the circulation and heart but not the BM. Moreover, we found decreased expression of IL-1β and IL-6, increased IL-10 expression and fewer apoptotic cardiomyocytes without changes in angiogenesis in the infarct area. Fractional shortening was enhanced 2 weeks after cell infusion but was similar to medium controls 16 weeks after MI. In vitro studies showed that BM MSCs increased the frequency of alternatively activated monocytes/macrophages, in part by MSC-mediated secretion of IL-10. Our data suggest a new mechanism for MSC-mediated enhancement of cardiac function, possibly via an IL-10 mediated switch from infiltration of pro-inflammatory to anti-inflammatory macrophages at the infarct site. Additional studies are warranted confirming the role of IL-10 and augmenting the anti-inflammatory effects of MSCs in cardiac regeneration.








Similar content being viewed by others
References
Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM (2005) Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA 102:11474–11479. doi:10.1073/pnas.0504388102
Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B (2007) Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 204:1057–1069. doi:10.1084/jem.20070075
Bennett S, Por SB, Cooley MA, Breit SN (1993) In vitro replication dynamics of human culture-derived macrophages in a long term serum-free system. J Immunol 150:2364–2371
Bréchot N, Gomez E, Bignon M, Khallou-Laschet J, Dussiot M, Cazes A, Alanio-Bréchot C, Durand M, Philippe J, Silvestre JS, Van Rooijen N, Corvol P, Nicoletti A, Chazaud B, Germain S (2008) Modulation of macrophage activation state protects tissue from necrosis during critical limb ischemia in thrombospondin-1-deficient mice. PLoS One 3:e3950. doi:10.1371/journal.pone.0003950
Burchfield JS, Iwasaki M, Koyanagi M, Urbich C, Rosenthal N, Zeiher AM, Dimmeler S (2008) Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection after myocardial infarction. Circ Res 103:203–211. doi:10.1161/CIRCRESAHA.108.178475
Chen L, Tredget EE, Wu PY, Wu Y (2008) Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3:e1886. doi:10.1371/journal.pone.0001886
Elenkov IJ, Webster E, Papanicolaou DA, Fleisher TA, Chrousos GP, Wilder RL (1998) Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J Immunol 161:2586–2593
Hu Y, Zhang H, Lu Y, Bai H, Xu Y, Zhu X, Zhou R, Ben J, Xu Y, Chen Q (2011) Class A scavenger receptor attenuates myocardial infarction-induced cardiomyocyte necrosis through suppressing M1 macrophage subset polarization. Basic Res Cardiol. doi:10.1007/s00395-011-0204-x
Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA (2001) Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest 107:1395–1402. doi:10.1172/JCI12150
Khan M, Mohsin S, Khan SN, Riazuddin S (2011) Repair of senescent myocardium by mesenchymal stem cells is dependent on the age of donor mice. J Cell Mol Med 15:1515–1520. doi:10.1111/j.1582-4934.2009.00998
Kleinbongard P, Heusch G, Schulz R (2010) TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther 127:295–314. doi:10.1016/j.pharmthera.2010.05.002
Krishnamurthy P, Rajasingh J, Lambers E, Qin G, Losordo DW, Kishore R (2009) IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ Res 104:e9–e18. doi:10.1161/CIRCRESAHA.108.188243
Lee GM, Fong S, Francis K, Oh DJ, Palsson BO (2000) In situ labeling of adherent cells with PKH26. In Vitro Cell Dev Biol Anim 36:4–6. doi:10.1290/1071-2690
Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ (2009) Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5:54–63. doi:10.1016/j.stem.2009.05.003
Lichtenauer M, Mildner M, Baumgartner A, Hasun M, Werba G, Beer L, Altmann P, Roth G, Gyöngyösi M, Podesser BK, Ankersmit HJ (2011) Intravenous intramyocardial injection of apoptotic white blood cell suspensions prevents ventricular remodelling by increasing elastin expression in cardiac scar tissue after myocardial infarction. Basic Res Cardiol 106:645–655. doi:10.1007/s00395-011-0173-0
Lyons AB (2000) Analysing cell division in vivo, in vitro using flow cytometric measurement of CFSE dye dilution. J Immunol Methods 243:147–154. doi:10.1016/S0022-1759(00)00231-3
Madonna R, Rokosh G, De Caterina R, Bolli R (2010) Hepatocyte growth factor/Met gene transfer in cardiac stem cells–potential for cardiac repair. Basic Res Cardiol 105:443–452. doi:10.1007/s00395-010-0102-7
Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, Nepomnaschy I, Costa H, Cañones C, Raiden S, Vermeulen M, Geffner JR (2010) Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One 5:e9252. doi:10.1371/journal.pone.0009252
Malefyt RW, Abrams J, Bennett B, Figdor CG, de Vries JE (1991) Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 174:1209–1220
Manning E, Pham S, Li S, Vazquez-Padron RI, Mathew J, Ruiz P, Salgar SK (2010) Interleukin-10 delivery via mesenchymal stem cells: a novel gene therapy approach to prevent lung ischemia-reperfusion injury. Hum Gene Ther 21:713–727. doi:10.1089/hum.2009.147
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25:677–686. doi:10.1016/j.it.2004.09.015
Marketou M, Kintsurashvili E, Papanicolaou KN, Lucero HA, Gavras I, Gavras H (2010) Cardioprotective effects of a selective B(2) receptor agonist of Bradykinin post-acute myocardial infarct. Am J Hypertens 23:562–568. doi:10.1038/ajh.2010.20
Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ (2007) The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204:3037–3047. doi:10.1084/jem.20070885
Oerlemans MI, Goumans MJ, van Middelaar B, Clevers H, Doevendans PA, Sluijter JP (2010) Active Wnt signaling in response to cardiac injury. Basic Res Cardiol 105:631–641. doi:10.1007/s00395-010-0100-9
Radin MJ, Holycross BJ, Dumitrescu C, Kelley R, Altschuld RA (2008) Leptin modulates the negative inotropic effect of interleukin-1beta in cardiac myocytes. Mol Cell Biochem 315:179–184. doi:10.1007/s11010-008-9805-6
Ritter M, Buechler C, Langmann T, Orso E, Klucken J, Schmitz G (2000) Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and anti-inflammatory stimuli. J Leukoc Biol 67:97–103. doi:10.1159/000028105
Sanganalmath SK, Abdel-Latif A, Bolli R, Xuan YT, Dawn B (2011) Hematopoietic cytokines for cardiac repair: mobilization of bone marrow cells and beyond. Basic Res Cardiol 106:709–733. doi:10.1007/s00395-011-0183-y
Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE (2005) Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells 23:220–229. doi:10.1634/stemcells.2004-0166
Schuh A, Liehn EA, Sasse A, Schneider R, Neuss S, Weber C, Kelm M, Merx MW (2009) Improved left ventricular function after transplantation of microspheres and fibroblasts in a rat model of myocardial infarction. Basic Res Cardiol 104:403–411. doi:10.1007/s00395-008-0763-7
Stein M, Keshav S, Harris N, Gordon S (1992) Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med 176:287–292
Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J (2005) Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol 175:342–349
Tolar J, LeBlanc K, Keating A, Blazar BR (2010) Hitting the right spot with mesenchymal stromal cells (MSCs). Stem Cells 28:1446–1455. doi:10.1002/stem.459
Wu J, Li J, Zhang N, Zhang C (2011) Stem cell-based therapies in ischemic heart diseases: a focus on aspects of microcirculation and inflammation. Basic Res Cardiol 106:317–324. doi:10.1007/s00395-011-0168-x
Acknowledgments
This research is supported by the Orsino Translational Research Laboratory at Princess Margaret Hospital. AK holds the Gloria and Seymour Epstein Chair in Cell Therapy and Transplantation at University Health Network and the University of Toronto.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dayan, V., Yannarelli, G., Billia, F. et al. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol 106, 1299–1310 (2011). https://doi.org/10.1007/s00395-011-0221-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-011-0221-9