Abstract
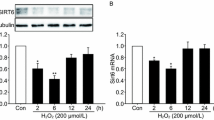
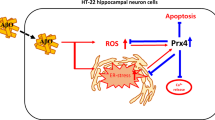
Oxidative stress is involved in the pathological processes of many neurodegenerative diseases. Protein modification by small ubiquitin-like modifiers (SUMOs) has been implicated in oxidative stress injury. By conjugating SUMOs to their selective protein substrates, SUMO ligases play critical roles in regulating functions of proteins involved in oxidative stress injury. In this study, we screened siRNAs to knockdown the SUMO ligase PIAS3 to assess its role in H2O2-induced injury in HT22 cells. H2O2 stimulation increased total protein SUMOylation, facilitated intracellular reactive oxygen species (ROS) release, increased cleaved caspase-3 levels, promoted p38 and JNK activation (phosphorylation), upregulated apoptosis, and decreased cell viability. The siRNA against PIAS3 329-347 (siPIAS3-329) markedly downregulated the protein expression of PIAS3 and reversed these effects, whereas siNC (negative control) had no effect. Our findings demonstrate that PIAS3-mediated SUMOylation facilitates oxidative stress injury and p38/JNK-mediated cell apoptosis and that PIAS3 is a potential target to protect against oxidative stress injury.




Similar content being viewed by others
Abbreviations
- SUMOs:
-
small ubiquitin-like modifiers
- ROS:
-
reactive oxygen species
- siPIAS3-329:
-
siRNA against PIAS3 329-347
- siNC:
-
negative control siRNA
- PIAS:
-
protein inhibitor of activated STAT
- PBS:
-
phosphate-buffered saline
- PI:
-
propidium iodide
- SD:
-
standard deviation
References
Pisoschi, A. M., & Pop, A. (2015). The role of antioxidants in the chemistry of oxidative stress: a review. European Journal of Medicinal Chemistry, 97, 55–74.
Sies, H. (2015). Oxidative stress: a concept in redox biology and medicine. Redox Biology, 4, 180–183.
Singh, A., Kukreti, R., Saso, L., & Kukreti, S. (2019). Oxidative stress: a key modulator in neurodegenerative diseases. Molecules, 24, 1583.
Teleanu, D. M., Niculescu, A. G., Lungu, I. I., Radu, C. I., Vladâcenco, O., Roza, E., Costăchescu, B., Grumezescu, A. M., & Teleanu, R. I. (2022). An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. International Journal of Molecular Sciences, 23, 5938.
Bai, R., Guo, J., Ye, X. Y., Xie, Y., & Xie, T. (2022). Oxidative stress: the core pathogenesis and mechanism of Alzheimer’s disease. Ageing Research Reviews, 77, 101619.
Kumar, A. & Ratan, RR. (2016). Oxidative stress and Huntingtons disease: the good, the bad, and the ugly. Journal Huntingtons Disease, 3, 217–237.
Dionísio, P. A., Amaral, J. D., & Rodrigues, C. M. P. (2021). Oxidative stress and regulated cell death in Parkinson’s disease. Ageing Research Reviews, 67, 101263.
Bouayed, J., Rammal, H., & Soulimani, R. (2009). Oxidative stress and anxiety: relationship and cellular pathways. Oxidative Medicine and Cellular Longevity, 2, 63–67.
Bhatt, S., Nagappa, A. N., & Patil, C. R. (2020). Role of oxidative stress in depression. Drug Discovery Today, 25, 1270–1276.
Chamorro, Á., Dirnagl, U., Urra, X., & Planas, A. M. (2016). Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurology, 15, 869–881.
Khatri, N., Thakur, M., Pareek, V., Kumar, S., Sharma, S., & Datusalia, A. K. (2018). Oxidative stress: major threat in traumatic brain injury. CNS Neurol Disord Drug Targets, 17, 689–695.
Henley, J. M., Carmichael, R. E., & Wilkinson, K. A. (2018). Extranuclear SUMOylation in neurons. Trends in Neurosciences, 41, 198–210.
Liu, F. Y., Liu, Y. F., Yang, Y., Luo, Z. W., Xiang, J. W., Chen, Z. G., Qi, R. L., Yang, T. H., Xiao, Y., Qing, W. J., & Li, D. W. (2017). SUMOylation in neurological diseases. Current Molecular Medicine, 16, 893–899.
Sahin, U., de Thé, H., & Lallemand-Breitenbach, V. (2022). Sumoylation in physiology, pathology and therapy. Cells, 11, 814.
Yau, T. Y., Molina, O., & Courey, A. J. (2020). SUMOylation in development and neurodegeneration. Development, 147, dev175703.
Stankovic-Valentin, N., & Melchior, F. (2018). Control of SUMO and Ubiquitin by ROS: Signaling and disease implications. Molecular Aspects of Medicine, 63, 3–17.
Pandey, D., Chen, F., Patel, A., Wang, C. Y., Dimitropoulou, C., Patel, V. S., Rudic, R. D., Stepp, D. W., & Fulton, D. J. (2011). SUMO1 negatively regulates reactive oxygen species production from NADPH oxidases. Arteriosclerosis, Thrombosis, and Vascular Biology, 31, 1634–1642.
Chhunchha, B., Fatma, N., Kubo, E., & Singh, D. P. (2014). Aberrant sumoylation signaling evoked by reactive oxygen species impairs protective function of Prdx6 by destabilization and repression of its transcription. FEBS Journal, 281, 3357–3381.
Chhunchha, B., Kubo, E., Fatma, N., & Singh, D. P. (2017). Sumoylation-deficient Prdx6 gains protective function by amplifying enzymatic activity and stability and escapes oxidative stress-induced aberrant Sumoylation. Cell Death & Disease, 8, e2525.
Kim, H. J., Yun, J., Lee, J., Hong, H., Jeong, J., Kim, E., Bae, Y. S., & Lee, K. J. (2011). SUMO1 attenuates stress-induced ROS generation by inhibiting NADPH oxidase 2. Biochemical and Biophysical Research Communications, 410, 555–562.
Creton, S., & Jentsch, S. (2010). SnapShot: the SUMO system. Cell, 143, e841.
Wilkinson, K. A., Nakamura, Y., & Henley, J. M. (2010). Targets and consequences of protein SUMOylation in neurons. Brain Research Reviews, 64, 195–212.
Henley, J. M., Craig, T. J., & Wilkinson, K. A. (2014). Neuronal SUMOylation: mechanisms, physiology, and roles in neuronal dysfunction. Physiological Reviews, 94, 1249–1285.
Krumova, P., & Weishaupt, J. H. (2013). Sumoylation in neurodegenerative diseases. Cellular and Molecular Life Sciences, 70, 2123–2138.
Leitao, B. B., Jones, M. C., & Brosens, J. J. (2011). The SUMO E3-ligase PIAS1 couples reactive oxygen species-dependent JNK activation to oxidative cell death. FASEB Journal, 25, 3416–3425.
Martin, S., Nishimune, A., Mellor, J. R., & Henley, J. M. (2007). SUMOylation regulates kainate-receptor-mediated synaptic transmission. Nature, 447, 321–325.
Ghosh, H., Auguadri, L., Battaglia, S., Simone Thirouin, Z., Zemoura, K., Messner, S., Acuña, M. A., Wildner, H., Yévenes, G. E., Dieter, A., Kawasaki, H., Hottiger, O. M., Zeilhofer, H. U., Fritschy, J. M., & Tyagarajan, S. K. (2016). Several posttranslational modifications act in concert to regulate gephyrin scaffolding and GABAergic transmission. Nature Communications, 7, 13365.
Du, C. P., Wang, M., Geng, C., Hu, B., Meng, L., Xu, Y., Cheng, B., Wang, N., Zhu, Q. J., & Hou, X. Y. (2020). Activity-induced SUMOylation of neuronal nitric oxide synthase is associated with plasticity of synaptic transmission and extracellular signal-regulated kinase 1/2 signaling. Antioxidants & Redox Signaling, 32, 18–34.
Meng, L., Du, C. P., Lu, C. Y., Zhang, K., Li, L., Yan, J. Z., & Hou, X. Y. (2021). Neuronal activity-induced SUMOylation of Akt1 by PIAS3 is required for long-term potentiation of synaptic transmission. FASEB Journal, 35, e21769.
Thirouin, Z. S., Figueiredo, M., Hleihil, M., Gill, R., Bosshard, G., McKinney, R. A., & Tyagarajan, S. K. (2022). Trophic factor BDNF inhibits GABAergic signaling by facilitating dendritic enrichment of SUMO E3 ligase PIAS3 and altering gephyrin scaffold. Journal of Biological Chemistry, 298, 101840.
Jiang, Y., Hu, L., Wang, B., Zhang, B., Shao, M., Meng, L., Xu, Y., Chen, R., Li, M., & Du, C. (2024). Disrupting PIAS3-mediated SUMOylation of MLK3 ameliorates poststroke neuronal damage and deficits in cognitive and sensorimotor behaviors. Cellular and Molecular Life Sciences, 81, 119.
Jiang, Y., Wang, B. X., Xie, Y., Meng, L., Li, M., & Du, C. P. (2023). MLK3 localizes mainly to the cytoplasm and promotes oxidative stress injury via a positive feedback loop. Cell Biochemistry and Biophysics, 81, 469–479.
Kwak, A. W., Kim, W. K., Lee, S. O., Yoon, G., Cho, S. S., Kim, K. T., Lee, M. H., Choi, Y. H., Lee, J. Y., Park, J. W., & Shim, J. H. (2023). Licochalcone B induces ROS-dependent apoptosis in oxaliplatin-resistant colorectal cancer cells via p38/JNK MAPK Signaling. Antioxidants, 12, 656.
Dröge, W. (2002). Free radicals in the physiological control of cell function. Physiological Reviews, 82, 47–95.
Bossis, G., & Melchior, F. (2006). Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Molecular Cell, 21, 349–357.
Stankovic-Valentin, N., Drzewicka, K., König, C., Schiebel, E., & Melchior, F. (2016). Redox regulation of SUMO enzymes is required for ATM activity and survival in oxidative stress. The EMBO Journal., 35, 1312–1329.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (32100769 and 82371401 to M.L. and 81100852 to C.-P.D.), the Natural Science Foundation of the Jiangsu Higher Education Institutions (20KJA310010 to C.-P.D.), and Xuzhou Medical University (D2020054 and JBGS202202 to M.L.).
Author information
Authors and Affiliations
Contributions
Baixue Wang performed biochemical research and analyzed data. Wenxin Qian and Kaiyue Chen assisted with cell culture and cell apoptotic detection. Meng Li and Caiping Du designed research, wrote the paper, and provided supervision and funding.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, B., Qian, W., Chen, K. et al. Knocking Down PIAS3 Reduces H2O2-induced Oxidative Stress Injury in HT22 Cells. Cell Biochem Biophys (2024). https://doi.org/10.1007/s12013-024-01292-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s12013-024-01292-y