Abstract
Cadmium (Cd) is a common environmental pollutant that leads to severe cardiotoxic hazards. Several studies were carried out to protect the myocardium against Cd-induced cardiotoxicity. Up till now, no researches evaluated the protective effect of dapagliflozin (DAP) against Cd induced cardiotoxicity. Thus, we aimed to explore the role of DAP in such model with deep studying of the involved mechanisms. 40 male Wistar albino rats were included in current study. Cd (5 mg/kg/day) was administered orally for 7 days to induce cardiotoxicity with or without co-administration of DAP in three different doses (2.5, 5, 10 mg/kg/day) orally for 7 days. Our data revealed that Cd could induce cardiotoxicity with significant increase in serum cardiac enzymes, heart weight, tissue malondialdehyde (MDA), tumor necrosis factor alpha (TNFα), nuclear factor kappa B (NFκB), toll like receptor2 (TLR2), interleukin 6 (IL6) and caspase3 immunoexpression with abnormal histopathological changes. In addition, Cd significantly decreased the level of heme oxygenase1 (HO1), nuclear factor erythroid 2-related factor 2 (Nrf2), signal transducer and activator of transcription (STAT3), reduced glutathione (GSH), glutathione peroxidase (GPx), and total antioxidant capacity (TAC). Co-administration of DAP could ameliorate Cd cardiotoxicity with significant improvement of the biochemical and histopathological changes. We found that DAP had protective properties against Cd induced cardiotoxicity and this may be due to its anti-oxidant, anti-inflammatory, anti-apoptotic properties and modulation of IL6/STAT3 and TLR2/TNFα-signaling pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is one of the most common toxic heavy metals that have severe cardiotoxic hazards especially on excessive exposure during cigarette smoking or food and water contamination. Cd has a great ability to accumulate in our bodies for very long period [1]. Several mechanisms mediate Cd cardiotoxicity such as induction of oxidative stress with release of free radicals, metabolic disturbance and dyslipidemia. Cardiotoxicity of Cd leads to atherosclerosis, coronary spasm, ischemic injury, myocardial infarction, hypertension, and stroke [1, 2]. Uncontrolled and excessive formation of free radicals is the main cause that stimulates oxidative stress and disturbs cellular function leading to membrane lipid peroxidation, DNA damage, and cell death. Furthermore, heart cells are highly sensitive to the released reactive oxygen species (ROS) and the anti-oxidative system of these cells is fully saturated by the endogenous oxidative metabolic products [1].
Up-regulation of the different inflammatory pathways is considered as another important cascade in mediating Cd cardiotoxicity. Stimulation of pro-inflammatory cytokines including tumor necrosis factor alpha (TNFα), interleukin6 (IL6) and nuclear factor kappa B (NFκB) is accompanied with enhancement of Janus kinase/signal transducer and activator of transcription (JAK/STAT) which is a critical pathway during cardiac injury [3,4,5]. STATs are considered as a family of protein that could regulate gene expression of angiogenesis, inflammation and apoptosis. This family is pre-stimulated with IL-6 and mediates different cardiac disorders as hypertrophy, myocardial infarction and heart failure [6,7,8]. Besides that, toll-like receptors (TLRs) control innate immunity and inflammation inside the cell and increase the gene expression of different pro-inflammatory cytokines such as TNFα and interleukins that have a critical role in myocardial tissue injuries. We can conclude that modulation of the TLR2/TNFα and IL6 /STAT pathways represents essential targets in controlling cardiac damage [9,10,11,12,13].
Sodium glucose co-transporter 2 (SGLT2) inhibitors including dapagliflozin (DAP) are the most recent oral anti-diabetic drug group. Its action is based on decreasing blood glucose level and inhibiting renal glucose reabsorption. Furthermore, DAP has non-glycemic effects including lowering of blood pressure, ability to control metabolic disorders and decreasing body weight [14,15,16,17]. DAP could decrease inflammatory cytokines including inflammasome, IL-1β and IL-6 but increase anti-inflammatory one; IL-10, increase the M2 (anti-inflammatory)/M1(pro-inflammatory) phenotypemacrophage ratio. In addition, DAP acts as an antioxidant and mediates M2 macrophage polarization through regulating STAT3 pathway with scavenging of reactive oxygen and nitrogen species followed by decrease of cardiac inflammation. Moreover; it controls the renal renin angiotensin aldosterone system, decreases cardiac and renal complications of diabetes, improves cardiac ischemia–reperfusion injury and doxorubicin cardiotoxicity depending on its pharmacological properties but till now there are no data regarding its role in Cd cardiotoxicity [18,19,20,21,22].
Increasing the critical need for finding new cardioprotective agents against Cd cardiotoxicity and the recently detected cardiopreserving properties of DAP guided us to evaluate its ability to control Cd cardiotoxicity and to investigate the different mechanisms mediating such effect including TLR2/TNFα and IL6/STAT3 pathways.
Materials and Methods
Chemicals
DAP and Cd were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Caspase3 (bsm-33199M) monoclonal antibody was from Bioss Antibodies Inc, USA. Moreover, ELISA kits of TLR-2 (Catalog # MBS264416), IL6 (Catalog # MBS269892), cardiac enzymes of creatine kinase-MB (CK-MB) (Catalog #MBS2515061), Lactate dehydrogenase (LDH) (Catalog # MBS043166), heme oxygenase 1(HO1) (Catalog # MBS764989), nuclear factor erythroid 2-related factor 2 (Nrf2) (Catalog # MBS1602926), troponin I (Catalog # MBS722833) were from My BioSource Co., San Diego, CA, USA. Total antioxidant capacity (TAC) (Catalog # TA2513) was obtained from Biodiagnostic, Egypt.
Animals and Experimental Design
40 male albino rats of Wistar species weighing about 200–250 g were included in current study. These animals were from Animal Research Centre, Giza, Egypt. Rats were kept in suitable conditions (3 rats/cage) and the duration of acclimatization was about 2 weeks with free access to chow and tap water. Our study was performed in accordance with the ethical standards of faculty of medicine, Minia University, Egypt in agreement with EU Directive 2010/63/EU for animal experiments.
Rats were randomly divided into 5 groups (n = 8 each).
DAP was dissolved in 1% carboxymethylcellulose and Cd was dissolved in drinking water, and then administered by oral gavage.
Group Ӏ received the vehicle orally for 7 days.
Group II was given Cd (5 mg/kg/day) orally [23] for 7 days.
Group III was administered DAP (2.5 mg/kg/day) orally and Cd (5 mg/kg/day) orally [23] for 7 days.
Group IV was given DAP (5 mg/kg/day) [24] and Cd (5 mg/kg/day) orally [23] for 7 days.
Group V received DAP (10 mg/kg/day) [25] and Cd (5 mg/kg/day) orally [23] for 7 days.
Preparation of Samples and Storage
Rats were euthanized on 7th day and each rat was anesthetized by i.p. injection of 20% Urethane hydrochloride (1 mg/kg). Arterial blood of each rat was collected from abdominal aorta then centrifuged at 5000 rpm for 15 min to obtain the sera (JanetzkiT30 centrifuge, Germany) for measuring cardiac enzymes and TAC. Each heart was excised, washed with saline and weighed. One part of each ventricle was fixed in 10% formalin and embedded in paraffin for further histopathological and immunohistochemical examination and another part was kept at − 80 °C. For preparing cardiac tissue homogenates specimens were homogenized in PBS 20%w/v using GLAS-Col homogenizer, USA then centrifuged at 3000 rpm for 20 min and the supernatant was separated and kept at − 80 °C till used.
Biochemical Measurements
Assessment of Cardiac Enzymes (Troponin I, CK-MB and LDH) in Serum
Serum cardiac enzymes were measured using the available commercial ELISA kits according to the manufacturers’ instruction based on sandwich ELISA immunoassay technique. The microtiter plate was pre-coated with a monoclonal antibody specific for each parameter then standards or samples were added to the microtiter plate wells and the detected enzyme bound to the antibody pre-coated wells. The enzyme–substrate reaction was terminated by addition of a sulphuric acid solution and the color change was measured spectrophotometrically. The color intensity was proportional to the concentration of the detected enzyme.
Evaluation of Oxidative Stress Parameters
We used colorimetric kit for measuring TAC and results were expressed as mmol/ml. MDA was the major product of lipoperoxidation that was detected colormetrically with the formation of MDA-thiobarbituric acid pink colored Schiff base adduct at 535 nm using 1, 1, 3, 3-tetramethoxypropane as standard and results were expressed as nmol/g tissue [26]. Measurement of GSH was based on binding of sulfhydryl group with Ellman’s reagent followed by formation of a yellow color that was detected colormetrically at 405 nm by Beckman DU-64 UV/VIS spectrophotometer, USA as µmol/g tissue [27].
Measurement of TLR2, HO1, Nrf2 and IL6
Detection of these markers was performed according to manufacturer’s instructions of the available ELISA kit depending on the double-sandwich ELISA technique. The pre-coated antibody of the measured parameters and the detecting antibody were labeled with biotin. Samples and biotin labeling antibody were added into ELISA plate wells and washed out then Avidin-peroxidase conjugates were added to ELISA wells. Finally, yellow color formed and the intensity of the color was positively correlated with the testing factors in samples.
Western Blotting of TNFα, NFκB, GPx and STAT3
Heart tissue homogenates about 50 μg of total proteins were boiled for 5 min with a buffer containing 2‑mercaptoethanol then loaded on 12% sodium dodecyl sulfate- polyacrylamide gel electrophoresis (SDS-PAGE) with running for 2 h at 100 V. After electrophoresis, proteins were blotted to polyvinylideneflouride (PVDF) membranes. Blocking step was for 1 h in a trisbuffered saline (TBS-T) and a blocking solution which contained 5% (w/v) non-fat milk and 0.05% Tween-20. Overnight incubation was at 4 °C with primary antibodies (1:1000) for rabbit anti-TNF-α (Catalog# 11948), rabbit anti-STAT3 (Catalog# 12640), rabbit anti-pY705 STAT3 (Catalog# 9145S), (Cell Signaling Technology, Danvers, MA, USA), anti-NFκB p65 antibody (Catalog# ab32536, Abcam, UK), anti-Glutathione Peroxidase 3/GPx-3 antibody (Catalog# ab256470, Abcam, UK) and anti-GAPDH antibody (Catalog# ab8245, Abcam, UK). Goat anti-rabbit polyclonal immunoglobulin conjugated with horseradish peroxidase (Cell Signaling Technology Inc., MA, USA) was used as a secondary antibody (1:5000) in blocking buffer. Bands were visualized by chemiluminescence, using an enhanced chemiluminescence kit (ECL, GE Healthcare, Chicago, IL, USA. Protein bands were evaluated densitometrically relative to GAPDH using Image J Software [28, 29].
Histopathological Examination
After sacrifice; part of each ventricle was prepared for histopathological examination, dissected, fixed in formaldehyde for 24 h and embedded in paraffin wax. Five µm sections were cut for hematoxylin and eosin stain and immunohistochemistry. The pathologist examined these slides in a blinded fashion using light microscopy (Olympus microscope, Japan). Scoring of the histopathological changes in different groups was assessed according to the degree of vascular congestion; hemorrhage; hemosiderosis; muscle striation; apoptosis and necrosis. The grades were as follows: score − normal, score + mild, score ++ moderate and score +++ severe [3].
Immunohistochemistry
Slides of the cardiac tissue specimens were de-paraffinized with xylene, hydrated in a descending graded ethyl alcohol then treated by 3% hydrogen peroxide for 30 min to block endogenous peroxides. Slides were washed in PBS solution and boiled for 20 min in citrate buffer (pH 6.0) by microwave for antigen retrieval [1, 3].
After rinse in PBS, caspase3 antibody was applied and incubated overnight then rinsed in PBS before applying the biotinylated secondary antibody for 30 min. The streptavidin–biotin complex reagent was added for 30 min after washing in PBS. For detection of brown color; 3, 3-diaminobenzidinetetra hydrochloride (DAB) was used. Slides were washed in distilled water, counterstained with hematoxylin, dehydrated in ethanol, cleared in xylene and covered slipped. The mean area percentage of active caspase 3 reaction was measured [30].
Statistical Analysis
Data of current model were analyzed using one-way ANOVA then Tukey’s multiple comparison test. The values were expressed as Mean ± SEM using GraphPad Prism software (version 5) for statistical analysis. The differences were considered as significant results when the P value < 0.05.
Results
Effect of DAP on Serum Cardiac Enzymes (Troponin I, CK-MB, LDH)
Cd (5 mg/kg/day) oral administration for 7 days led to significant increase in serum cardiac enzymes level (troponin I, CK-MB, LDH) compared to control group. Co-administration of DAP significantly ameliorated this effect compared to Cd given group alone in dose dependent manner (Table 1).
Effect of DAP on the Evaluated Oxidative Stress Parameters (MDA, GSH, HO1, Nrf2 and TAC) in Cd Induced Cardiotoxicity
Cd group significantly increased MDA but decreased GSH, HO1, Nrf2 and TAC compared to control group. DAP plus Cd given groups significantly decreased MDA but increased GSH, HO1, Nrf2 and TAC compared to Cd administered group alone. High dose of DAP was more protective than lower doses (Table 2).
Effect of DAP on Heart Weights, TLR2, IL6 in Cd Induced Cardiotoxicity
ELISA measurement of TLR2, IL6 showed a significant increase in their tissue levels in Cd given group compared to control group. However, co-administration of DAP could significantly ameliorate these changes in comparison to Cd cardiotoxic group in dose dependent manner and high dose was more protective than lower doses (Table 3).
Heart weights significantly increased in Cd cardiotoxic group compared to control group. On the contrary, Cd plus DAP showed significant decrease in heart weight compared to Cd given group.
Western Blotting Results for Measuring TNFα, NFκB, GPx and P-STAT3
Results revealed a significant increase of TNFα and NFκB but a decrease of GPx and P-STAT3 level in Cd cardiotoxic group compared to control one but co-administration of DAP decreased the tissue level of TNFα and NFκB but increased GPx and P-STAT3 compared to Cd given group alone (Figs. 1a, b, 2a, b).
Evaluation of TNFα and NFκB by western blotting. TNFα and NFκB significantly increased in cadmium given group compared to control group. However, co-administration of dapagliflozin significantly decreased TNFα and NFκB levels in comparison to cadmium given group alone. Values are representation of 8 observations as Mean ± SEM. Results are considered significantly different when P < 0.05. aSignificant difference compared to control, bSignificant difference compared to cadmium given group, csignificant difference compared to dapagliflozin high dose (10 mg/kg/day) plus cadmium given group
P-STAT3, GPx results using western blotting. Data revealed significant decrease of P-STAT3 and GPx levels in cadmium given group compared to control group. However, co-administration of dapagliflozin showed significant increase of P-STAT3 and GPx levels in comparison with cadmium given group alone. Values are representation of 8 observations as Mean ± SEM. Results are considered significantly different when P < 0.05.aSignificant difference compared to control, bSignificant difference compared to cadmium given group, cSignificant difference compared to dapagliflozin high dose (10 mg/kg/day) plus cadmium given group
Histopathological Evaluation Results (Fig. 3)
Histopathological evaluation results. a Photomicrographs of myocardial sections of control group shows striated branching cardiac muscle fibres with acidophilic cytoplasm and central oval vesicular nucleus (arrows) separated by blood capillaries (C). b and c longitudinal and cross myocardial sections of cadmium cardiotoxic group show fragmented necrotic cardiac muscle fibers (*). Some fibers have pyknotic nuclei and hyperacidophilic cytoplasm (black arrows), marked congested dilated blood capillary (C), inflammatory cells infiltration (circles) and hemorrhage (H). d represents low dose dapagliflozin treated group that shows fragmented necrotic pale stained cardiac muscle fibers (*), some fibers have pyknotic nuclei and hyperacidophilic cytoplasm (black arrows), marked congested dilated blood capillary (C) and inflammatory cells infiltration (circles). e represents moderate dose dapagliflozin treated group showing cardiac muscle fibres appear more or less normal, still few fibres have pyknotic nuclei (arrows). Moreover, mild congested blood capillaries (C) and few scattered inflammatory cells (circle) are noticed. f of high dose dapagliflozin treated group showing cardiac muscle fibres appear more or less normal, still few scattered inflammatory cells (circle) are noticed [H&E, ×400]
Myocardial sections of control group showed normal structure with striated branching cardiac muscle fibres, acidophilic cytoplasm, central oval vesicular nuclei and the cardiomyocytes were separated by blood capillaries (Fig. 3a). The longitudinal and cross myocardial sections of Cd cardiotoxic group showed fragmented necrotic cardiac muscle fibers, some fibers had pyknotic nuclei and hyperacidophilic cytoplasm (apoptotic morphology). Moreover, there were markedly congested and dilated blood capillaries. Inflammatory cell infiltration and areas of hemorrhage were also noticed (Fig. 3b, c). In the low dose DAP treated group, necrotic pale stained cardiac muscle fibers, scattered fibers with apoptotic morphology (pyknotic nuclei and hyperacidophilic cytoplasm), dilated blood capillary and inflammatory cell infiltration were also evident (Fig. 3d). Moderate dose DAP treated group showed the cardiac muscle fibres with more or less normal features but still few fibres had pyknotic nuclei. Also, mild congested blood capillaries and few scattered inflammatory cells were observed (Fig. 3e). Interestingly, there was marked improvement in high dose DAP treated group as the cardiac muscle fibres appeared more or less normal with few scattered inflammatory cells in-between (Fig. 3f). These data were supported by scoring of the histopathological findings (Table 4).
Evaluation of Cleaved Caspase3 Immunoexpression (Fig. 4)
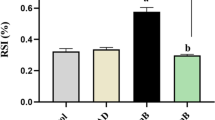
Evaluation of cleaved caspase3 immunoexpression. Photomicrographs of heart tissue reveal immunohistochemical expression of activated caspase3 a control group shows no expression either in the cardiomyocytes (*) or endothelium lining of blood capillaries (arrow), b cadmium cardiotoxic group shows high expression in the cytoplasm of the cardiomyocyte (*) and in the endothelial lining of the blood capillaries (arrows), c low dose dapagliflozin treated group shows moderate expression in the cytoplasm of the cardiomyocyte (*) and in the endothelial lining of the blood capillaries (arrows), d moderate dose dapagliflozin treated group shows faint expression in few cardiomyocytes (*). Still most of the capillary endothelium shows positive expression (arrows), e high dose dapagliflozin treated group shows negative expression in the cardiomyocytes (*). Few endothelial cells show positive expression (arrow), (active caspase3 immunohistochemistry ×400). f Semiquantitative analysis of cleaved caspase3 immunoexpression. Results showed significant increase in its immunoexpression in cadmium given group in comparison to normal control group. However, dapagliflozin plus cadmium showed significant decrease in cleaved caspase3 immunoexpression compared to cadmium administered group alone. Values are representation of 8 observations as Mean ± SEM. Results are considered significantly different when P < 0.05.aSignificant difference compared to control, bSignificant difference compared to cadmium given group, cSignificant difference compared to dapagliflozin high dose (10 mg/kg/day) plus cadmium given group
Sections of control heart tissue, immunostained with activated caspase3 showed negative expression either in the cardiomyocytes or endothelial lining of the blood capillaries. Heart tissues of Cd cardiotoxic group had high expression in the cytoplasm of the cardiomyocyte and the endothelial lining of the blood capillaries. Moderate expression was also noticed in the cytoplasm of the cardiomyocyte and the endothelium of the low dose DAP treated group. In the moderate dose DAP treated group, the expression was faint and localized to few cardiomyocytes, although most of the capillary endothelium showed positive expression. The cardiomyocytes of the high dose DAP treated group showed negative expression, while few endothelium cells showed positive expression.
Semiquantitative Analysis of Cleaved Caspase3 Immunoexpression (Fig. 4f)
Results showed a significant increase of cleaved caspase3 immunoexpression in Cd cardiotoxic group compared to control group. However, there was a significant decrease in its immunoexpression in DAP co-administered groups in dose dependent manner.
Discussion
Cd is a well-known environmental contaminant, cardiotoxic, and carcinogenic heavy metal [2, 31]. Our model is the first step in exploring the cardioprotective properties of DAP in Cd induced cardiac injury with studying of the different mechanisms that mediate such effect. Cd induced cardiac injury was detected in our results in form of significant increase of cardiac enzymes, heart weights, MDA, TLR2, IL6, caspase3, TNFα, NFκB but significant decrease in P-STAT3, GPx, GSH, TAC with cardiotoxic features in the histopathological examination in form of hemorrhage, inflammation, necrosis and fibrosis.
Indiscriminate exposure to Cd causes generation of free radicals and oxidative dysfunction that leads to lipid peroxidation. This associates with alteration in the antioxidant defense mechanism followed by oxidative injury of proteins, DNA, and lipids that is found in our model as significant increase in MDA levels; the most reliable marker for evaluating oxidative stress and lipid peroxidation [32,33,34,35,36]. In addition, other oxidative stress markers including lipid hydroperoxides and protein carbonyls increased in Cd induced toxicity and they have a critical role in mediating such injury.
Antioxidant enzymes are considered as the first line of defense against the oxidative challenges to protect cell membrane and to keep the cellular integrity and prevent the pathogenesis of different degenerative diseases. Superoxide is converted by superoxide dismutase to hydroxyl radicals and hydrogen peroxide then GPx utilizes GSH to reduce hydrogen peroxide with formation of oxidized glutathione and H2O. During oxidative stress, the level of enzymatic antioxidant enzymes notably GPx as well as the non-enzymatic antioxidant, GSH, decreases due to interaction with the excessively released free radicals [5, 19, 29, 37,38,39,40]. The same was observed in our results as there is general reduction in the measured GSH, GPx and TAC in Cd treated rats and this is in accordance with previous studies [31, 39,40,41,42].
Nrf2/HO1 pathway is also highly essential in evaluating Cd cardiotoxicity. Nrf2 is kept inactive in the cytoplasm binding with keap under healthy conditions. However, upon stimulation of oxidative stress, it travels into the nucleus and attaches to its DNA sequence followed by stimulation of gene transcription of several antioxidants including HO1 that catalyzes the rate-limiting step in the process of heme catabolism leading to anti-inflammatory, anti-oxidant, anti-apoptotic effects; it also controls cell proliferation and differentiation [43]. Our model reveals significant downregulation of Nrf2/HO1 pathway and the same was detected with others [43, 44].
Oxidative stress induces cell membrane lipid peroxidation and damage with release of the intracellular cardiac enzymes and increasing their serum level including troponin I, CK-MB and LDH. Troponin I is one of the contractile cardiac apparatus that activates sliding of actin over myosin. It is a specific diagnostic marker for detecting cardiac injury. Moreover, CK-MB is widely distributed in skeletal muscles all over the body and highly sensitive to the occurrence of cardiac injury [1, 2, 34,35,36]. This is in accordance with our data that revealed marked increase in troponin I, CK-MB and LDH in Cd cardiotoxic group.
Initiation of inflammation is another essential signaling cascade in mediating Cd cardiotoxicity. We evaluated two essential pathways; IL6/STAT3 and TLR2/TNFα which have a great role in mediating different tissue injuries. We found significant disturbance of both pathways in Cd given group reflecting occurrence of inflammation. In addition, JAK/STAT signaling cascade is considered as a critical inflammatory pathway in cardiac injury that stimulates release of other inflammatory and apoptotic mediators including TNFα and NFκB [45, 46]. One of STATs family is STAT3 which is controlled by IL6 and highly expressed in many heart cells including cardiomyocytes, fibroblasts, smooth muscle cells, endothelial cells, and inflammatory cells. Disturbances in IL6/STAT3 pathway could enhance Cd induced cardiac damage. Moreover, STAT3 is a crucial regulator of various intracellular events and it can regulate autophagy by promoting its mitochondrial localization. However, downregulation of STAT3 leads to cell apoptosis and damage. Notably, recent studies have been carried out based on the cardioprotective effect of STAT3 [46,47,48]. Besides that, there is another inflammatory signaling pathway; TLR2/TNFα that has an essential role in Cd induced toxicities. Toll-like receptors enhance further release of TNFα and other interleukins followed by activation of an important inflammatory and apoptotic molecule; NFκB which is highly involved in Cd induced cardiotoxicity [2, 10,11,12,13, 49]. This is in accordance with our current findings that showed significant increases of TNFα, NFκB and IL6 in Cd given group compared to normal control group.
Occurrence of oxidative stress and inflammation is followed by stimulation of the apoptotic cascade that is evaluated by measuring caspases family proteins. Programmed cell death occurs due to imbalance between anti-apoptotic and pro-apoptotic factors upon stimulation with the released free radicals and other pro-inflammatory agents as TNFα and NFκB. This is followed by releasing of several caspases such as caspase3 which is the most important indicator of apoptosis. Moreover, caspase3 is essential in both extrinsic and intrinsic pathways [8, 11,12,13]. The extrinsic apoptotic pathway is activated upon binding of a certain ligand to the death receptor followed by activation of caspase8 then either initiates apoptosis directly by activating executioner caspase, or activates the intrinsic apoptotic pathway. This signaling cascade can be activated through various cellular stresses leading to cytochrome c release from the mitochondria with formation of APAF1, cytochrome c, ATP, and caspase9 complex (the apoptosome) which initiates apoptosis by cleaving executioner caspases [50,51,52]. We measured caspase3 immunoexpression as an apoptotic marker in Cd given group and results revealed significant increase in its expression reflecting occurrence of apoptosis due to Cd administration and this is in accordance with previous studies [1, 2, 34,35,36].
Sodium glucose co-transporter 2 inhibitor is a class of oral anti-diabetic drugs recently used for lowering blood glucose level in type 2 diabetic patients. This drug group acts on proximal tubule in the kidneys and decreases renal glucose reabsorption with increasing glucose excretion [53]. Despite the great benefits of SGLT-2 inhibitors, their different mechanisms of cardioprotection are still incompletely understood. Possible actions are proposed to be systemic effects via controlling metabolic disturbance and hemodynamic properties; natriuresis and glycosuria followed by decreasing cardiac preload and afterload without changing heart rate.
SGLT is highly expressed in the heart and scientists suggested that this drug class has an indirect effect on the cardiovascular system via ameliorating volume overload, decreasing blood pressure and controlling renin angiotensin aldosterone system associated with decreasing cardiac remodeling and hypertrophy with reduction of blood pressure [54]. Besides that, DAP could decrease calcium overload, attenuating inflammation and different pro-inflammatory and inflammatory cytokines including inflammasome/caspase1/IL1β pathway, IL6, macrophage infiltration and fibrosis. This effect is accompanied with regulation of BAX/Bcl2 apoptotic pathway and downregulation of apoptosis as a result of reducing ROS [55]. In addition, DAP could decrease M1macrophage polymerization and superoxide free radicals with activation of STAT3 followed by reduction of inflammation, oxidative stress, apoptosis and regulation of mitochondrial function [20, 56]. However, Cd induces mitochondrial dysfunction, increases M1 macrophage polymerization but decreases M2 with up-regulation of oxidative stress and inflammation [37, 38].
Several studies found that this drug group could ameliorate cardiac morphologic changes including cardiac hypertrophy, fibrosis, heart failure and decrease the infarcted size. Moreover; DAP could improve systolic and diastolic LV function in cases of diabetic cardiomyopathy and control cardiac arrhythmia in I/R injury. Furthermore, they decreased cardiac preload and afterload with diminishing intracellular Na+ and Ca2+loading that is mostly detected in heart failure [20].
This in agreement with our findings which reveal that co-administration of DAP could markedly ameliorate Cd induced biochemical and histopathological changes. Our results found significant decrease and normalization of cardiac enzymes level including serum CK-MB, LDH and Troponin I. Moreover, there is marked regulation of oxidant/antioxidant misbalance including oxidative stress parameter, MDA level but increases tissue anti-oxidants as GSH and GPx with elevation of the whole TAC causing protection of the cell membrane and preventing release of cardiac enzymes. In addition, DAP succeeded in modulating IL6/STAT3 and TLR2/TNFα pathway reflecting its ability to suppress both inflammatory and apoptotic processes as found in previous models [20, 56, 57]. Caspase3; an essential apoptotic marker, decreased significantly on co-administration of DAP with Cd in comparison to Cd given group alone confirming its anti-apoptotic effect with marked improvement of the histopathological features.
This cardioprotective properties of DAP was already discussed in other models of cardiac injury including diabetic cardiomyopathy, ischemia reperfusion models, doxorubicin cardiotoxicity, myocardial infarction and heart failure [20, 21, 57,58,59]. These effects were based upon anti-oxidant, anti-inflammatory and anti-apoptotic properties of DAP and its ability to regulate renin angiotensin aldosterone system and keep the myocardium. Our model may give a promise to consider DAP as cardiac protector against Cd cardiotoxicity.
Conclusion
Dapagliflozin could significantly reduce cadmium cardiotoxicity by various mechanisms including anti-oxidant, ant-inflammatory and anti-apoptotic properties via modulating IL6/STAT3 and TLR2/TNFα pathways. Further studies are highly encouraged to evaluate the cardioprotective role of dapagliflozin in cadmium cardiotoxic patients.
Data Availability
All data are available in prism files as supplementary material.
Code Availability
Not applicable.
Change history
01 March 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s12012-023-09787-5
References
Sarmiento-Ortega, V. E., Brambila, E., Flores-Hernández, J. Á., Díaz, A., Peña-Rosas, U., & Moroni-González, D. (2018). The NOAEL metformin dose is ineffective against metabolic disruption induced by chronic cadmium exposure in wistar rats. Toxics, 6(3), E55.
Refaie, M. M. M., El-Hussieny, M., Bayoumi, A. M. A., & Shehata, S. (2019). Mechanisms mediating the cardioprotective effect of carvedilol in cadmium induced cardiotoxicity. Role of eNOS and HO1/Nrf2 pathway. Environmental Toxicology Pharmacology, 70, 103198.
Chang, H., Zhao, F., Xie, X., et al. (2019). PPARα suppresses Th17 cell differentiation through IL-6/STAT3/RORγt pathway in experimental autoimmune myocarditis. Experimental Cell Research, 375(1), 22–30.
Shati, A. A., & El-Kott, A. F. (2019). Acylated ghrelin prevents doxorubicin-induced cardiac intrinsic cell death and fibrosis in rats by restoring IL-6/JAK2/STAT3 signaling pathway and inhibition of STAT1. Naunyn Schmiedebergs Arch Pharmacology, 392(9), 1151–1168.
Harrison, D. A. (2012). The Jak/STAT pathway. Cold Spring Harbor Perspectives in Biology, 4(3), a011205.
Heinrich, P. C., Behrmann, I., Haan, S., Hermanns, H. M., Müller-Newen, G., & Schaper, F. (2003). Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochemical Journal, 374(1), 1–20.
Lee, T. M., Chang, N. C., & Lin, S. Z. (2017). Dapagliflozin, a selective SGLT2 inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radical Biology and Medicine, 104, 298–310.
Refaie, M. M. M., Shehata, S., Bayoumi, A. M. A., El-Tahawy, N. F. G., & Abdelzaher, W. Y. (2022). The IL-6/STAT signaling pathway and PPARα are involved in mediating the dose-dependent cardioprotective effects of fenofibrate in 5-fluorouracil-induced cardiotoxicity. Cardiovascular Drugs & Therapy., 36(5), 817–827.
El-Zayat, S. R., Sibaii, H., & Mannaa, F. A. (2019). Toll-like receptors activation, signaling, and targeting: An overview. Bulletin of the National Research Centre., 43(1), 187.
Arslan, F., Smeets, M. B., O’Neill, L. A., et al. (2010). Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation, 121(1), 80–90.
Cristofaro, P., & Opal, S. M. (2003). The Toll-like receptors and their role in septic shock. Expert Opinion on Therapeutic Targets, 7(5), 603–612.
Ehrentraut, H., Weber, C., Ehrentraut, S., et al. (2011). The toll-like receptor 4-antagonist eritoran reduces murine cardiac hypertrophy. European Journal of Heart Failure, 13(6), 602–610.
Yu, L., & Feng, Z. (2018). The role of toll-like receptor signaling in the progression of heart failure. Mediators of Inflammation, 2018, 9874109.
Abdel-Wahab, A. F., Bamagous, G. A., Al-Harizy, R. M., et al. (2018). Renal protective effect of SGLT2 inhibitor dapagliflozin alone and in combination with irbesartan in a rat model of diabetic nephropathy. Biomedicine & Pharmacotherapy., 103, 59–66.
Tanajak, P., Sa-Nguanmoo, P., Sivasinprasasn, S., et al. (2018). Cardioprotection of dapagliflozin and vildagliptin in rats with cardiac ischemia-reperfusion injury. Journal of Endocrinololgy, 236(2), 69–84.
Erdogan, M. A., Yusuf, D., Christy, J., et al. (2018). Highly selective SGLT2 inhibitor dapagliflozin reduces seizure activity in pentylenetetrazol-induced murine model of epilepsy. BMC Neurology Journal, 18(1), 81.
Oguma, T., Nakayama, K., Kuriyama, C., et al. (2015). Intestinal sodium glucose cotransporter 1 inhibition enhances glucagon-like peptide-1 secretion in normal and diabetic rodents. Journal of Pharmacology and Experimental Therapeutics, 354, 279–289.
Lahnwong, S., Palee, S., Apaijai, N., et al. (2020). Acute dapagliflozin administration exerts cardioprotective effects in rats with cardiac ischemia/reperfusion injury. Cardiovascular Diabetology, 19(1), 91.
Shin, S. J., Chung, S., Kim, S. J., et al. (2016). Effect of sodium-glucose co-transporter 2 inhibitor, dapagliflozin, on renal renin-angiotensin system in an animal model of type 2 diabetes. PLoS ONE, 11(11), e0165703.
Lahnwong, S., Chattipakorn, S. C., & Chattipakorn, N. (2018). Potential mechanisms responsible for cardioprotective effects of sodium–glucose co-transporter 2 inhibitors. Cardiovascular Diabetology, 17(10), 1.
Joubert, M., Jagu, B., Montaigne, D., et al. (2017). The sodium-glucose cotransporter 2 inhibitor dapagliflozin prevents cardiomyopathy in a diabetic lipodystrophic mouse model. Diabetes, 66(4), 1030–1040.
Jaikumkao, K., Pongchaidecha, A., Chueakula, N., et al. (2018). Dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, slows the progression of renal complications through the suppression of renal inflammation, endoplasmic reticulum stress and apoptosis in prediabetic rats. Diabetes, Obesity and Metabolism, 20(11), 2617–2626.
Mukherjee, R., Banerjee, S., Joshi, N., Singh, P. K., Baxi, D., & Ramachandran, A. V. (2011). A combination of melatonin and alpha lipoic acid has greater cardioprotective effect than either of them singly against cadmium-induced oxidative damage. Cardiovascular Toxicology, 11(1), 78–88.
Arab, H. H., Al-Shorbagy, M. Y., & Saad, M. A. (2021). Activation of autophagy and suppression of apoptosis by dapagliflozin attenuates experimental inflammatory bowel disease in rats: Targeting AMPK/mTOR, HMGB1/RAGE and Nrf2/HO-1 pathways. Chemico-Biological Interactions, 335, 109368.
Kingir, Z. B., Özdemir Kural, Z. N., Cam, M. E., et al. (2019). Effects of dapagliflozin in experimental sepsis model in rats. Ulus Travma Acil Cerrahi Derg, 25(3), 213–221.
Buege, J. A., & Aust, S. D. (1978). Microsomal lipid peroxidation. Methods in Enzymology, 52, 302–310.
Moron, M. S., Depierre, J. W., & Mannervik, B. (1979). Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochimica et Biophysica Acta, 582(1), 67–78.
Chadha, S., Wang, L., Hancock, W. W., & Beier, U. H. (2019). Sirtuin-1 in immunotherapy: A Janus-headed target. Journal of Leukocyte Biology, 106(2), 337–343.
Chen, Y., Zhang, Y., Huo, Y., Wang, D., & Hong, Y. (2016). Adrenomedullin mediates tumor necrosis factor-alpha-induced responses in dorsal root ganglia in rats. Brain Research, 1644, 183–191.
Hamza, A. A., Fikry, E. M., Abdallah, W., & Amin, A. (2018). Mechanistic insights into the augmented effect of bone marrow mesenchymal stem cells and thiazolidinediones in streptozotocin-nicotinamide induced diabetic rat. Scientific Reports, 8(1), 9827.
Milton Prabu, S., Muthumani, M., & Shagirtha, K. (2013). Quercetin potentially attenuates cadmium induced oxidative stress mediated cardiotoxicity and dyslipidemia in rats. European Review for Medical and Pharmacological Sciences, 17(5), 582–595.
Nishiyama, S., Saito, N., Konishi, Y., Abe, Y., & Kusumi, K. (1990). Cardiotoxicity in magnesium-deficient rats fed cadmium. Journal of Nutritional Science and Vitaminology, 36(1), 33–44.
Oyinloye, B. E., Ajiboye, B. O., Ojo, O. A., Nwozo, S. O., & Kappo, A. P. (2016). Cardioprotective and antioxidant influence of aqueous extracts from Sesamum indicum seeds on oxidative stress induced by cadmium in Wistar rats. Pharmacognosy Magazine, 12(Suppl 2), S170–S174.
Priya, L. B., Baskaran, R., Elangovan, P., Dhivya, V., Huang, C. Y., & Padma, V. V. (2017). Tinospora cordifolia extract attenuates cadmium-induced biochemical and histological alterations in the heart of male Wistar rats. Biomedicine & Pharmacotherapy, 87, 280–287.
Jamall, I. S., & Smith, J. C. (1985). Effects of cadmium on glutathione peroxidase, superoxide dismutase, and lipid peroxidation in the rat heart: A possible mechanism of cadmium cardiotoxicity. Toxicolog and Applied Pharmacology, 80(1), 33–42.
Alpsoy, S., Kanter, M., Aktas, C., et al. (2014). Protective effects of onion extract on cadmium-induced oxidative stress, histological damage, and apoptosis in rat heart. Biological Trace Element Research, 159(1–3), 297–303.
Yao, Y., Zhao, X., Zheng, S., Wang, S., Liu, H., & Xu, S. (2021). Subacute cadmium exposure promotes M1 macrophage polarization through oxidative stress-evoked inflammatory response and induces porcine adrenal fibrosis. Toxicology, 461, 152899.
Xu, S., Xiaojing, L., Xinyue, S., Wei, C., Honggui, L., & Shiwen, X. (2021). Pig lung fibrosis is active in the subacute CdCl 2 exposure model and exerts cumulative toxicity through the M1/M2 imbalance. Ecotoxicology and Environmental Safety, 225, 112757.
Lubos, E., Loscalzo, J., & Handy, D. E. (2011). Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxidants & Redox Signaling, 15(7), 1957–1997.
Esworthy, R. S., Ho, Y. S., & Chu, F. F. (1997). The Gpx1 gene encodes mitochondrial glutathione peroxidase in the mouse liver. Archives of Biochemistry and Biophysics, 340, 59–63.
Esworthy, R. S., Yang, L., Frankel, P. H., & Chu, F. F. (2005). Epithelium-specific glutathione peroxidase, Gpx2, is involved in the prevention of intestinal inflammation in selenium-deficient mice. Journal of Nutrition, 135, 740–745.
Darwish, W. S., Chen, Z., Li, Y., Wu, Y., Chiba, H., & Hui, S. P. (2020). Identification of cadmium-produced lipid hydroperoxides, transcriptomic changes in antioxidant enzymes, xenobiotic transporters, and pro-inflammatory markers in human breast cancer cells (MCF7) and protection with fat-soluble vitamins. Environmental Science and Pollution Research, 27(2), 1978–1990.
Refaie, M. M. M., El-Hussieny, M., Bayoumi, A. M. A., & Shehata, S. (2019). Mechanisms mediating the cardioprotective effect of carvedilol in cadmium induced cardiotoxicity. Role of eNOS and HO1/Nrf2 pathway. Environmental Toxicology Pharmacology, 70, 103198.
Bashir, N., Shagirtha, K., Manoharan, V., & Miltonprabu, S. (2019). The molecular and biochemical insight view of grape seed proanthocyanidins in ameliorating cadmium-induced testes-toxicity in rat model: Implication of PI3K/Akt/Nrf-2 signaling. Bioscience Reports, 39(1), BSR20180515.
Zhang, J., Sun, Z., Lin, N., et al. (2020). Fucoidan from Fucus vesiculosus attenuates doxorubicin-induced acute cardiotoxicity by regulating JAK2/STAT3-mediated apoptosis and autophagy. Biomedicine & Pharmacotherapy, 130, 110534.
Zhang, L., Liu, L., & Li, X. (2020). MiR-526b-3p mediates doxorubicin-induced cardiotoxicity by targeting STAT3 to inactivate VEGFA. Biomedicine & Pharmacotherapy, 123, 109751.
Pipicz, M., Demján, V., Sárközy, M., & Csont, T. (2018). Effects of cardiovascular risk factors on cardiac STAT3. International Journal of Molecular Sciences, 19(11), 3572.
Chu, X., Zhang, Y., Xue, Y., et al. (2020). Crocin protects against cardiotoxicity induced by doxorubicin through TLR-2/NF-kappaB signal pathway in vivo and vitro. International Immunopharmacology, 84, 106548.
Kabel, A. M., & Elkhoely, A. A. (2017). Targeting proinflammatory cytokines, oxidative stress, TGF-beta1 and STAT-3 by rosuvastatin and ubiquinone to ameliorate trastuzumab cardiotoxicity. Biomedicine & Pharmacotherapy, 93, 17–26.
McIlwain, D. R., Berger, T., & Mak, T. W. (2013). Caspase functions in cell death and disease. Cold Spring Harbor Perspectives in Biology, 5(4), a008656.
Kang, T. B., Ben-Moshe, T., Varfolomeev, E. E., Pewzner-Jung, Y., Yogev, N., Jurewicz, A., Waisman, A., Brenner, O., Haffner, R., Gustafsson, E., et al. (2004). Caspase-8 serves both apoptotic and nonapoptotic roles. Journal of Immunology, 173, 2976–2984.
Brenner, D., & Mak, T. W. (2009). Mitochondrial cell death effectors. Current Opinion in Cell Biology, 21, 871–877.
Chang, Y. K., Choi, H., Jeong, J. Y., et al. (2016). Dapagliflozin, SGLT2 inhibitor, attenuates renal ischemia-reperfusion injury. PLoS ONE, 11(7), e0158810.
Dekkers, C. C. J., Petrykiv, S., Laverman, G. D., Cherney, D. Z., Gansevoort, R. T., & Heerspink, H. J. L. (2018). Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes, Obesity and Metabolism, 20(8), 1988–1993.
Shibusawa, R., Yamada, E., Okada, S., et al. (2019). Dapagliflozin rescues endoplasmic reticulum stress-mediated cell death. Scientific Reports, 9(1), 9887.
Chang, W. T., Lin, Y. W., Ho, C. H., Chen, Z. C., Liu, P. Y., & Shih, J. Y. (2021). Dapagliflozin suppresses ER stress and protects doxorubicin-induced cardiotoxicity in breast cancer patients. Archives of Toxicology, 95(2), 659–671.
Zhang, N., Feng, B., Ma, X., Sun, K., Xu, G., & Zhou, Y. (2019). Dapagliflozin improves left ventricular remodeling and aorta sympathetic tone in a pig model of heart failure with preserved ejection fraction. Cardiovascular Diabetology, 18(1), 107.
Lim, G. B. (2020). Dapagliflozin reduces left ventricular mass. Nature Reviews Cardiology, 17(9), 540.
Garnock-Jones, K. P. (2017). Saxagliptin/dapagliflozin: A review in type 2 diabetes mellitus. Drugs, 77(3), 319–330.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Dr. MMMR and Dr. SS selected the point, performed the experimental part, wrote the manuscript and sent it for publication. Dr. RAR performed and wrote the histopathology and immunohistochemistry. Dr. MAF performed and wrote the part of western blotting analysis. All authors revised the final version of the manuscript and agreed for publishing it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical Approval
Animal handling, medications, and animal sacrifice were carried out according to the guidelines of the experimental animals care and approved by the Institutional Ethical Committee, Faculty of Medicine, Minia University, Egypt in agreement with the NIH Guide for taking care and use of laboratory animals. Approval No. 20:3/2021.
Informed Consent
Not applicable.
Research Involving Human Participants and/or Animals
Animals.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Handling Editor: Travis Knuckles.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1007/s12012-023-09787-5
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Refaie, M.M.M., Rifaai, R.A., Fawzy, M.A. et al. RETRACTED ARTICLE: Dapagliflozin Guards Against Cadmium-Induced Cardiotoxicity via Modulation of IL6/STAT3 and TLR2/TNFα Signaling Pathways. Cardiovasc Toxicol 22, 916–928 (2022). https://doi.org/10.1007/s12012-022-09768-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-022-09768-0