Abstract
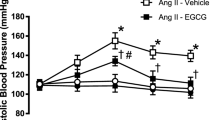
Oxidative stress plays an important role in the pathogenesis of hypertension. Epigallocatechin-3-O-gallate (EGCG) is the main polyphenol present in green tea and is known for its potent antioxidant and anti-inflammatory properties. In the present study, we hypothesize that EGCG attenuates oxidative stress in the paraventricular nucleus of hypothalamus (PVN), thereby decreasing the blood pressure and sympathetic activity in renovascular hypertensive rats. After renovascular hypertension was induced in male Sprague-Dawley rats by the two-kidney one-clip (2K-1C) method, the rats received bilateral PVN infusion of EGCG (20 μg/h) or vehicle via osmotic minipump for 4 weeks. Our results were shown as follows: (1) Hypertension induced by 2K-1C was associated with the production of reactive oxygen species in the PVN; (2) chronic infusion of EGCG in the PVN decreased stress-related NAD(P)H oxidase subunit gp91phox and NOX-4 and increased the activity of antioxidant enzymes (SOD-1), also balanced the content of cytokines (IL-1β, IL-6, IL-10 and MCP-1) in the PVN, and attenuated the level of norepinephrine in plasma of 2K-1C rats. Our findings provide strong evidence that PVN infusion of EGCG inhibited renovascular hypertension progression through its potent anti-oxidative and anti-inflammatory activity in the PVN.






Similar content being viewed by others
References
Alderman, M. H., & Ogihara, T. (2007). Global challenge for overcoming high blood pressure: Fukuoka Statement, 19 October 2006. Journal of Hypertension, 25, 727.
Lopez, A. D., Mathers, C. D., Ezzati, M., Jamison, D. T., & Murray, C. J. (2006). Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet, 367, 1747–1757.
Kearney, P. M., Whelton, M., Reynolds, K., Muntner, P., Whelton, P. K., & He, J. (2005). Global burden of hypertension: Analysis of worldwide data. Lancet, 365, 217–223.
Behradmanesh, S., & Nasri, H. (2013). Association of serum calcium with level of blood pressure in type 2 diabetic patients. Journal of Nephropathology, 2, 254–257.
Lenfant, C., Chobanian, A. V., Jones, D. W., & Roccella, E. J. (2003). Seventh report of the Joint National Committee on the prevention, detection, evaluation, and treatment of high blood pressure (JNC 7): Resetting the hypertension sails. Hypertension, 41, 1178–1179.
Mittal, B. V., & Singh, A. K. (2010). Hypertension in the developing world: Challenges and opportunities. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation, 55, 590–598.
Savoia, C., Burger, D., Nishigaki, N., Montezano, A., & Touyz, R. M. (2011). Angiotensin II and the vascular phenotype in hypertension. Expert Reviews in Molecular Medicine, 13, e11.
Belmonte, S. L., & Blaxall, B. C. (2011). G protein coupled receptor kinases as therapeutic targets in cardiovascular disease. Circulation Research, 109, 309–319.
Zubcevic, J., Waki, H., Raizada, M. K., & Paton, J. F. (2011). Autonomic-immune-vascular interaction: an emerging concept for neurogenic hypertension. Hypertension, 57, 1026–1033.
Muller, D. N., Kvakan, H., & Luft, F. C. (2011). Immune-related effects in hypertension and target-organ damage. Current Opinion in Nephrology and Hypertension, 20, 113–117.
Touyz, R. M. (2005). Molecular and cellular mechanisms in vascular injury in hypertension: Role of angiotensin II. Current Opinion in Nephrology and Hypertension, 14, 125–131.
Baylis, C. (2012). Nitric oxide synthase derangements and hypertension in kidney disease. Current Opinion in Nephrology and Hypertension, 21, 1–6.
Rodrigo, R., Gonzalez, J., & Paoletto, F. (2011). The role of oxidative stress in the pathophysiology of hypertension. Hypertension Research: Official Journal of the Japanese Society of Hypertension, 34, 431–440.
Wilcox, C. S. (2005). Oxidative stress and nitric oxide deficiency in the kidney: A critical link to hypertension? American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 289, R913–R935.
Vaziri, N. D. (2004). Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Current Opinion in Nephrology and Hypertension, 13, 93–99.
Touyz, R. M. (2004). Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension, 44, 248–252.
Droge, W. (2002). Free radicals in the physiological control of cell function. Physiological Reviews, 82, 47–95.
Kakihana, T., Nagata, K., & Sitia, R. (2012). Peroxides and peroxidases in the endoplasmic reticulum: Integrating redox homeostasis and oxidative folding. Antioxidants and Redox Signaling, 16, 763–771.
Laurindo, F. R., Pescatore, L. A., & Fernandes Dde, C. (2012). Protein disulfide isomerase in redox cell signaling and homeostasis. Free Radical Biology and Medicine, 52, 1954–1969.
Lee, J., Giordano, S., & Zhang, J. (2012). Autophagy, mitochondria and oxidative stress: Cross-talk and redox signalling. The Biochemical Journal, 441, 523–540.
Touyz, R. M., Briones, A. M., Sedeek, M., Burger, D., & Montezano, A. C. (2011). NOX isoforms and reactive oxygen species in vascular health. Molecular Interventions, 11, 27–35.
Vaziri, N. D., & Rodriguez-Iturbe, B. (2006). Mechanisms of disease: Oxidative stress and inflammation in the pathogenesis of hypertension. Nature Clinical Practice Nephrology, 2, 582–593.
Touyz, R. M., & Schiffrin, E. L. (2004). Reactive oxygen species in vascular biology: Implications in hypertension. Histochemistry and Cell Biology, 122, 339–352.
Touyz, R. M., & Briones, A. M. (2011). Reactive oxygen species and vascular biology: Implications in human hypertension. Hypertension Research: Official Journal of the Japanese Society of Hypertension, 34, 5–14.
Chen, D. D., Dong, Y. G., Yuan, H., & Chen, A. F. (2012). Endothelin 1 activation of endothelin A receptor/NADPH oxidase pathway and diminished antioxidants critically contribute to endothelial progenitor cell reduction and dysfunction in salt-sensitive hypertension. Hypertension, 59, 1037–1043.
Huang, B. S., Zheng, H., Tan, J., Patel, K. P., & Leenen, F. H. (2011). Regulation of hypothalamic renin–angiotensin system and oxidative stress by aldosterone. Experimental Physiology, 96, 1028–1038.
Schupp, N., Kolkhof, P., Queisser, N., Gartner, S., Schmid, U., Kretschmer, A., et al. (2011). Mineralocorticoid receptor-mediated DNA damage in kidneys of DOCA-salt hypertensive rats. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 25, 968–978.
Kimura, M., Umegaki, K., Kasuya, Y., Sugisawa, A., & Higuchi, M. (2002). The relation between single/double or repeated tea catechin ingestions and plasma antioxidant activity in humans. European Journal of Clinical Nutrition, 56, 1186–1193.
Frei, B., & Higdon, J. V. (2003). Antioxidant activity of tea polyphenols in vivo: Evidence from animal studies. The Journal of Nutrition, 133, 3275S–3284S.
Higdon, J. V., & Frei, B. (2003). Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Critical Reviews in Food Science and Nutrition, 43, 89–143.
Thomas, R., & Kim, M. H. (2005). Epigallocatechin gallate inhibits HIF-1α degradation in prostate cancer cells. Biochemical and Biophysical Research Communications, 334, 543–548.
Zaveri, N. T. (2006). Green tea and its polyphenolic catechins: Medicinal uses in cancer and noncancer applications. Life Sciences, 78, 2073–2080.
Nakachi, K., Matsuyama, S., Miyake, S., Suganuma, M., & Imai, K. (2000). Preventive effects of drinking green tea on cancer and cardiovascular disease: Epidemiological evidence for multiple targeting prevention. BioFactors, 13, 49–54.
Kang, W. S., Lim, I. H., Yuk, D. Y., Chung, K. H., Park, J. B., Yoo, H. S., et al. (1999). Antithrombotic activities of green tea catechins and (–)-epigallocatechin gallate. Thrombosis Research, 96, 229–237.
Townsend, P. A., Scarabelli, T. M., Pasini, E., Gitti, G., Menegazzi, M., Suzuki, H., et al. (2004). Epigallocatechin-3-gallate inhibits STAT-1 activation and protects cardiac myocytes from ischemia/reperfusion-induced apoptosis. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 18, 1621–1623.
Lorenz, M., Wessler, S., Follmann, E., Michaelis, W., Dusterhoft, T., Baumann, G., et al. (2004). A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. The Journal of Biological Chemistry, 279, 6190–6195.
Baluchnejadmojarad, T., & Roghani, M. (2011). Chronic epigallocatechin-3-gallate ameliorates learning and memory deficits in diabetic rats via modulation of nitric oxide and oxidative stress. Behavioural Brain Research, 224, 305–310.
Baba, Y., Sonoda, J. I., Hayashi, S., Tosuji, N., Sonoda, S., Makisumi, K., et al. (2012). Reduction of oxidative stress in liver cancer patients by oral green tea polyphenol tablets during hepatic arterial infusion chemotherapy. Experimental and Therapeutic Medicine, 4, 452–458.
Kochi, T., Shimizu, M., Terakura, D., Baba, A., Ohno, T., Kubota, M., et al. (2014). Non-alcoholic steatohepatitis and preneoplastic lesions develop in the liver of obese and hypertensive rats: Suppressing effects of EGCG on the development of liver lesions. Cancer Letters, 342, 60–69.
Wang, M. H., Chang, W. J., Soung, H. S., & Chang, K. C. (2012). (–)-Epigallocatechin-3-gallate decreases the impairment in learning and memory in spontaneous hypertension rats. Behavioural Pharmacology, 23, 771–780.
Hodgson, J. M., & Croft, K. D. (2010). Tea flavonoids and cardiovascular health. Molecular Aspects of Medicine, 31, 495–502.
Babu, P. V., & Liu, D. (2008). Green tea catechins and cardiovascular health: An update. Current Medicinal Chemistry, 15, 1840–1850.
Potenza, M. A., Marasciulo, F. L., Tarquinio, M., Tiravanti, E., Colantuono, G., Federici, A., et al. (2007). EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. American Journal of Physiology. Endocrinology and metabolism, 292, E1378–E1387.
Su, Q., Qin, D. N., Wang, F. X., Ren, J., Li, H. B., Zhang, M., et al. (2014). Inhibition of reactive oxygen species in hypothalamic paraventricular nucleus attenuates the renin-angiotensin system and proinflammatory cytokines in hypertension. Toxicology and Applied Pharmacology, 276, 115–120.
Kang, Y. M., Ma, Y., Zheng, J. P., Elks, C., Sriramula, S., Yang, Z. M., et al. (2009). Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovascular Research, 82, 503–512.
Cardinale, J. P., Sriramula, S., Mariappan, N., Agarwal, D., & Francis, J. (2012). Angiotensin II-induced hypertension is modulated by nuclear factor-kappa B in the paraventricular nucleus. Hypertension, 59, 113–121.
Zimmerman, M. C., Lazartigues, E., Sharma, R. V., & Davisson, R. L. (2004). Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circulation Research, 95, 210–216.
Kang, Y. M., Chen, J. Y., Ouyang, W., Qiao, J. T., Reyes-Vazquez, C., & Dafny, N. (2004). Serotonin modulates hypothalamic neuronal activity. The International Journal of Neuroscience, 114, 299–319.
Han, Y., Fan, Z. D., Yuan, N., Xie, G. Q., Gao, J., De, W., et al. (2011). Superoxide anions in the paraventricular nucleus mediate the enhanced cardiac sympathetic afferent reflex and sympathetic activity in renovascular hypertensive rats. Journal of Applied Physiology, 110, 646–652.
Zhu, G. Q., Xu, Y., Zhou, L. M., Li, Y. H., Fan, L. M., Wang, W., et al. (2009). Enhanced cardiac sympathetic afferent reflex involved in sympathetic overactivity in renovascular hypertensive rats. Experimental Physiology, 94, 785–794.
Kang, Y. M., He, R. L., Yang, L. M., Qin, D. N., Guggilam, A., Elks, C., et al. (2009). Brain tumour necrosis factor-alpha modulates neurotransmitters in hypothalamic paraventricular nucleus in heart failure. Cardiovascular Research, 83, 737–746.
Qi, J., Zhang, D. M., Suo, Y. P., Song, X. A., Yu, X. J., Elks, C., et al. (2013). Renin–angiotensin system modulates neurotransmitters in the paraventricular nucleus and contributes to angiotensin II-induced hypertensive response. Cardiovascular Toxicology, 13, 48–54.
Li, H. B., Qin, D. N., Ma, L., Miao, Y. W., Zhang, D. M., Lu, Y., et al. (2014). Chronic infusion of lisinopril into hypothalamic paraventricular nucleus modulates cytokines and attenuates oxidative stress in rostral ventrolateral medulla in hypertension. Toxicology and Applied Pharmacology, 279, 141–149.
Gao, L., Wang, W., Li, Y. L., Schultz, H. D., Liu, D., Cornish, K. G., et al. (2005). Sympathoexcitation by central ANG II: Roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. American Journal of Physiology. Heart and circulatory Physiology, 288, H2271–H2279.
Kang, Y. M., Zhang, Z. H., Johnson, R. F., Yu, Y., Beltz, T., Johnson, A. K., et al. (2006). Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circulation Research, 99, 758–766.
Agarwal, D., Welsch, M. A., Keller, J. N., & Francis, J. (2011). Chronic exercise modulates RAS components and improves balance between pro- and anti-inflammatory cytokines in the brain of SHR. Basic Research in Cardiology, 106, 1069–1085.
Coote, J. H. (2007). Landmarks in understanding the central nervous control of the cardiovascular system. Experimental Physiology, 92, 3–18.
Joyner, M. J., Charkoudian, N., & Wallin, B. G. (2008). A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Experimental Physiology, 93, 715–724.
Martinez-Maldonado, M. (1991). Pathophysiology of renovascular hypertension. Hypertension, 17, 707–719.
Katholi, R. E., Whitlow, P. L., Winternitz, S. R., & Oparil, S. (1982). Importance of the renal nerves in established two-kidney, one clip Goldblatt hypertension. Hypertension, 4, 166–174.
Cohn, H. I., Harris, D. M., Pesant, S., Pfeiffer, M., Zhou, R. H., Koch, W. J., et al. (2008). Inhibition of vascular smooth muscle G protein-coupled receptor kinase 2 enhances α1D-adrenergic receptor constriction. American Journal of Physiology. Heart and circulatory physiology, 295, H1695–H1704.
Campos, R. R., Oliveira-Sales, E. B., Nishi, E. E., Boim, M. A., Dolnikoff, M. S., & Bergamaschi, C. T. (2011). The role of oxidative stress in renovascular hypertension. Clinical and Experimental Pharmacology and Physiology, 38, 144–152.
Chen, A. D., Zhang, S. J., Yuan, N., Xu, Y., De, W., Gao, X. Y., et al. (2011). Angiotensin AT1 receptors in paraventricular nucleus contribute to sympathetic activation and enhanced cardiac sympathetic afferent reflex in renovascular hypertensive rats. Experimental Physiology, 96, 94–103.
Maliszewska-Scislo, M., Chen, H., Augustyniak, R. A., Seth, D., & Rossi, N. F. (2008). Subfornical organ differentially modulates baroreflex function in normotensive and two-kidney, one-clip hypertensive rats. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 295, R741–R750.
Kishi, T., & Hirooka, Y. (2012). Oxidative stress in the brain causes hypertension via sympathoexcitation. Frontiers in Physiology, 3, 335.
Wu, K. L., Chan, S. H., & Chan, J. Y. (2012). Neuroinflammation and oxidative stress in rostral ventrolateral medulla contribute to neurogenic hypertension induced by systemic inflammation. Journal of Neuroinflammation, 9, 212.
Zimmerman, M. C., Sharma, R. V., & Davisson, R. L. (2005). Superoxide mediates angiotensin II-induced influx of extracellular calcium in neural cells. Hypertension, 45, 717–723.
Shi, Z., Gan, X. B., Fan, Z. D., Zhang, F., Zhou, Y. B., Gao, X. Y., et al. (2011). Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiologica (Oxf), 203, 289–297.
Taubert, D., Roesen, R., & Schomig, E. (2007). Effect of cocoa and tea intake on blood pressure: A meta-analysis. Archives of Internal Medicine, 167, 626–634.
Liu, P. L., Liu, J. T., Kuo, H. F., Chong, I. W., & Hsieh, C. C. (2014). Epigallocatechin gallate attenuates proliferation and oxidative stress in human vascular smooth muscle cells induced by interleukin-1beta via heme oxygenase-1. Mediators of Inflammation, 2014, 523684.
Steffen, Y., Gruber, C., Schewe, T., & Sies, H. (2008). Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Archives of Biochemistry and Biophysics, 469, 209–219.
Rodrigo, R. (2012). Prevention of postoperative atrial fibrillation: Novel and safe strategy based on the modulation of the antioxidant system. Frontiers in Physiology, 3, 93.
Simic, D. V., Mimic-Oka, J., Pljesa-Ercegovac, M., Savic-Radojevic, A., Opacic, M., Matic, D., et al. (2006). Byproducts of oxidative protein damage and antioxidant enzyme activities in plasma of patients with different degrees of essential hypertension. Journal of Human Hypertension, 20, 149–155.
Roghani, M., & Baluchnejadmojarad, T. (2009). Chronic epigallocatechin-gallate improves aortic reactivity of diabetic rats: underlying mechanisms. Vascular Pharmacology, 51, 84–89.
Antonello, M., Montemurro, D., Bolognesi, M., Di Pascoli, M., Piva, A., Grego, F., et al. (2007). Prevention of hypertension, cardiovascular damage and endothelial dysfunction with green tea extracts. American Journal of Hypertension, 20, 1321–1328.
Khalaf, A. A., Moselhy, W. A., & Abdel-Hamed, M. I. (2012). The protective effect of green tea extract on lead induced oxidative and DNA damage on rat brain. Neurotoxicology, 33, 280–289.
Acknowledgments
This study was supported by National Basic Research Program of China (No. 2012CB517805) and National Natural Science Foundation of China (Nos. 91439120, 81471471, 81170248, 81370356, 31171095). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None of the listed authors has any financial or other interests that could be of conflict.
Additional information
Qiu-Yue Yi, Jie Qi and Xiao-Jing Yu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yi, QY., Qi, J., Yu, XJ. et al. Paraventricular Nucleus Infusion of Epigallocatechin-3-O-Gallate Improves Renovascular Hypertension. Cardiovasc Toxicol 16, 276–285 (2016). https://doi.org/10.1007/s12012-015-9335-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-015-9335-x