Abstract
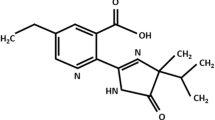
Pollutants including insecticides have been recently reported to be a risk factor involved in various diseases. Permethrin, a member of the family of synthetic pyrethroids, is widely used as insecticide in agriculture and other domestic applications. To investigate possible cardiotoxicity, we had examined different concentrations of permethrin on the freshly isolated rat heart cells using the alkaline comet assay. A significant difference in % tail DNA between all concentrations of permethrin (5, 10, 20 μM) and vehicle (control) without enzymes and with Fpg-treated cells were measured. The results indicated that permethrin induced oxidative damage to purine bases in the heart cells. Pyrimidines oxidation was evaluated using Endonuclease III (Endo III), but the results did not reveal any significant changes. After permethrin exposure, cells were studied to evaluate their DNA repair capacity. A complete DNA repair at 10 and 20 μM was measured after 30 and 60 min of repair intervals. Significant change in plasma membrane fluidity at different depths of bilayer was measured following permethrin treatment. Membrane fluidity in the hydrophilic–hydrophobic region was reduced, while the hydrophobic inner resulted more fluid following permethrin treatment of heart cells. This work points to standardize conditions applicable to ex vivo cells following in vivo treatment in order to study the cardiotoxicity of insecticide.





Similar content being viewed by others
References
Bradberry, S. M., Cage, S. A., Proudfoot, A. T., & Vale, J. A. (2005). Poisoning due to pyrethroids. Toxicological reviews, 24(2), 93–106.
Keikotlhaile, B. M., Spanoghea, P., & Steurbauta, W. (2010). Effects of food processing on pesticide residues in fruits and vegetables: A meta-analysis approach. Food and Chemical Toxicology, 48(1), 1–6.
Weston, D. P., You, J., & Lydy, M. J. (2004). Distribution and toxicity of sediment-associated pesticides in agricultural-dominated water bodies of California’s central valley. Environmental Science and Technology, 38(10), 2752–2759.
Agnihotri, N.P. (1999) Pesticide safety evaluation and monitoring (pp.13–14). Division of Agriculture Chemicals. New Delhi: Indian Agricultural Research Institute.
Kumari, B., Madan, V. K., Kumar, R., & Kathpal, T. S. (2002). Monitoring of seasonal vegetables for pesticide residue. Environmental Monitoring and Assessment, 74, 263–270.
Kumari, B., Kumar, R., Madan, V. K., Singh, R., Singh, J., & Kathpal, T. S. (2003). Magnitude of pesticidal contamination in winter vegetables from Hisar, Hariana. Environmental Monitoring and Assessment, 87, 311–318.
Kumari, B., Madan, V. K., & Kathpal, T. S. (2006). Monitoring of pesticide residues in fruits. Environmental Monitoring and Assessment, 123, 407–412.
Hussain, S., Masud, T., & Ahad, K. (2002). Determination of pesticides residues in selected varieties of mango. Asian network for scientific information. Pakistan Journal of Nutrition, 1, 41–42.
Fortin, M. C., Carrier, G., & Bouchard, M. (2008). Concentrations versus amounts of biomarkers in urine: A comparison of approaches to assess pyrethroid exposure. Environ Health, 7, 55–68.
Leng, G., Gries, W., & Selim, S. (2006). Biomarker of pyrethrum exposure. Toxicology Letters, 162, 195–201.
Saieva, C., Aprea, C., Tumino, R., Masala, G., Salvini, S., Frasca, G., et al. (2004). Twenty-four-hour urinary excretion of ten pesticide metabolites in healthy adults in two different areas of Italy (Florence and Ragusa). The Science of the Total Environment, 332, 71–80.
Spencer, C. I., Yuill, K. H., Borg, J. J., Hancox, J. C., & Kozlowski, R. Z. (2001). Actions of pyrethroid insecticides on sodium currents, action potentials and contractile rhythm in isolated mammalian ventricular myocytes and perfused hearts. The Journal of pharmacology and experimental therapeutics, 298, 1067–1082.
Berlin, J. R., Akera, T., Brody, T. M., & Matsumara, F. (1984). Changes in neuromuscular transmission of guinea pig vas deferens produced by decamethrin treatment. European Journal of Pharmacology, 98, 313–322.
Luty, S., Latuszynska, J., Halliop, J., Tochman, A., Obuchowska, D., Przylepa, E., et al. (1998). Toxicity of dermally applied alpha-cypermethrin in rats. Annals of Agricultural and Environmental Medicine, 5, 109–116.
Mueller, C. F., Laude, K., McNally, J. S., & Harrison, D. G. (2005). ATVB in focus: Redox mechanisms in blood vessels. Arteriosclerosis, Thrombosis, and Vascular Biology, 25, 274–278.
Van Gaal, L. F., Mertens, I. L., & De Block, C. E. (2006). Mechanisms linking obesity with cardiovascular disease. Nature, 444, 875–880.
Droge, W. (2002). Free radicals in the physiological control of cell function. Comprehensive overview of the reactions involved in generating and scavenging reactive oxygen radicals, good insight into antioxidant mechanisms of action. Physiological Reviews, 82, 47–95.
You, H., Kim, G., Kim, Y., Chun, Y., Park, J., Chung, M. H., et al. (2000). Increased 8-hydroxyguanine formation and endonuclease activity for its repair in ischemic-reperfused hearts of rats. Journal of Molecular and Cellular Cardiology, 32, 1053–1059.
Golubnitschaja, O., Moenkemann, H., Kim, K., & Mozaffari, M. S. (2003). DNA damage and expression of checkpoint genes p21 (WAF1/CIP1) and 14–3-3 sigma in taurine-deficient cardiomyocytes. Biochemical Pharmacology, 66, 511–517.
Berwick, M., & Vineis, P. (2000). Markers of DNA repair and susceptibility to cancer in humans: An epidemiologic review. Journal of the National Cancer Institute, 92, 874–897.
Kastan, M. B., & Bartek, J. (2004). Cell-cycle checkpoints and cancer. Nature, 432, 316–323.
Singh, N. P., McCoy, M. T., Tice, R. R., & Schneider, E. L. (1988). A simple technique for quantification of low levels of DNA damage in individual cells. Experimental Cell Research, 175, 184–191.
Hellman, B., Brodin, D., Andersson, M., Dahlman-Wright, K., Isacsson, U., Brattstrom, D., et al. (2005). Radiation-induced DNA-damage and gene expression profiles in human lung cancer cell lines with different radiosensitivity. Experimental oncology, 27(2), 102–107.
Report of FAO. Pesticide residues in food 1981, Monographs paper 42, Geneva, November–December 1981.
Kakko, I., Toimela, T., & Tahti, H. (2003). The synaptosomal membrane bound ATPase as a target for the neurotoxic effects of pyrethroids, permethrin and cypermethrin. Chemosphere, 51, 475–480.
Grosman, N., & Diel, F. (2005). Influence of pyrethroids and piperonyl butoxide on the Ca (2+)-ATPase activity of rat brain synaptosomes and leukocyte membranes. International Immunopharmacology, 5, 263–270.
Gabbianelli, R., Falcioni, M. L., Cantalamessa, F., & Nasuti, C. (2009). Permethrin induces Endo III and Fpg lymphocyte DNA damage and change in monocyte respiratory burst in rats. Journal of Applied Toxicology, 29(4), 317–322.
Van der Wees, C. G., Vreeswijk, M. P., Persoon, M., Van der Laarse, A., Van Zeeland, A. A., & Mullenders, L. H. (2003). Deficient global genome repair of UV-induced cyclobutane pyrimidine dimers in terminally differentiated myocytes and proliferating fibroblasts from the rat heart. DNA Repair, 2, 1297–1308.
Pool-Zobel, B. L., Guigas, C., Klein, R. G., Neudecker, C. H., Renner, H. W., & Schnezer, P. (1993). Assessment of genotoxic effect by lindane. Food and Chemical Toxicology, 31, 271–283.
Moretti, M., Villarini, M., Scasselati-Sforzolini, G., Santoni, A. M., Fedeli, D., & Falcioni, G. (1998). Extent of DNA damage in density-separated trout erythrocytes assessed by “Comet” assay. Mutation Research, 397, 353–360.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. L. (1951). Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry, 193, 265–275.
Shinitzky, M., & Barenholz, Y. (1978). Fluidity parameters of lipid regions determined by fluorescence polarization. Biochimica et Biophysica Acta, 515, 367–394.
Parasassi, T., De Stasio, G., D’Ubaldo, A., & Gratton, E. (1990). Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophysical Journal, 57, 1179–1186.
Parasassi, T., De Stasio, G., Ravagnan, G., Rusch, R. M., & Gratton, E. (1991). Quantitation of lipid phases in phospholipids vesicles by the generalized polarization of Laurdan fluorescence. Biophysical Journal, 60, 179–189.
Kumaravel, T. S., & Jha, A. N. (2006). Reliable comet assay measurements for detecting DNA damage induced by ionising radiation and chemicals. Mutation Research, 605, 7–16.
Fairbain, D. W., Olive, P. L., & O’Neill, K. L. (1995). The comet assay: A comprehensive review. Mutation Research, 339, 37–59.
Tice, R. R., Agurell, E., Anderson, D., Burlinson, B., Hartmann, A., Kobayashi, H., et al. (2000). Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environmental and Molecular Mutagenesis, 35, 206–221.
Olive, P. L., Johnston, P. J., Banath, J. P., & Durand, R. E. (1998). The comet assay: A new method to examine heterogeneity associated with solid tumours. Nature Medicine, 4, 103–105.
Collins, A. R. (2004). The comet assay for DNA damage and repair. Molecular Biotechnology, 26, 249–261.
Nishi, E. E., Campos, R. R., Bergamaschi, C. T., De Almeida, V. R., & Ribeiro, D. A. (2010). Vitamin C prevents DNA damage induced by renovascular hypertension in multiple organs of Wistar rats. Human & Experimental Toxicology, 29(7), 593–599.
Lenzi, P., Frenzilli, G., Gesi, M., Ferrucci, M., Lazzeri, G., Fornai, F., et al. (2003). DNA damage associated with ultrastructural alterations in rat myocardium after loud noise exposure. Environmental Health Perspectives, 111, 467–471.
Smith, C. C., O’Donovan, M. R., & Martin, E. A. (2006). HOGG1 recognizes oxidative damage using the comet assay with greater specificity than FPG or ENDOIII. Mutagenesis, 21, 185–190.
Halliwell, B., & Aruoma, O. I. (1991). DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Letters, 281, 9–19.
Ferrari, R., Agnoletti, L., Comini, L., Gaia, G., Bachetti, T., Cargnoni, A., et al. (1998). Oxidative stress during myocardial ischemia and heart failure. European Heart Journal Supplements, B19, 2–11.
Tomascik-Cheeseman, L. M., Coleman, M. A., Marchetti, F., Nelson, D. O., Kegelmeyer, L. M., Nath, J., et al. (2004). Differential basal expression of genes associated with stress response, damage control, and DNA repair among mouse tissues. Mutation Research, 561, 1–14.
Caroline, G. C., Van der Wees, P. G., Vreeswijk, M., Persoonmoud, A., Van der Laarse, A., Zeeland, H. F., et al. (2003). Deficient global genome repair of UV-induced cyclobutane pyrimidine dimers in terminally differentiated myocytes and proliferating fibroblasts from the rat heart. DNA Repair, 2, 1297–1308.
Marguet, D., Lenne, P. F., Rigneault, H., & He, H. T. (2006). Dynamics in the plasma membrane: How to combine fluidity and order. The EMBO Journal, 25(15), 3446–3457.
Cester, N., Rabini, R. A., Salvolini, E., Staffolani, R., Curatola, A., Pugnaloni, A., et al. (1996). Activation of endothelial cells during insulin-dependent diabetes mellitus: A biochemical and morphological study. European Journal of Clinical Investigation, 26, 569–573.
Sojcic, Z., Toplak, H., Zuehlke, R., Honegger, U. E., Buhlmann, R., & Wiesmann, U. N. (1992). Cultured human skin fibroblasts modify their plasma membrane lipid composition and fluidity according to growth temperature suggesting homeoviscous adaptation at hypothermic (30°) but not at hyperthermic (40°) temperatures. Biochimica et Biophysica Acta, 1104, 31–37.
Ohyashiki, T., Karino, T., Suzuki, S., & Matsui, K. (1996). Effect of aluminum ion on Fe (21)-induced lipid peroxidation in phospholipid liposomes under acidic conditions. Journal of Biochemistry, 120, 895–900.
Gabbianelli, R., Falcioni, M. L., Nasuti, C., Cantalamessa, F., Imada, I., & Inoue, M. (2009). Effect of permethrin insecticide on rat polymorphonuclear neutrophil. Chemico-Biological Interactions, 182(23), 245–252.
Kale, M. (1999). Lipid Peroxidation and Antioxidant Enzymes in Rat Tissues in Pyrethroid Toxicity: Possible Involvement of Reactive Oxygen Species. Journal of Nutritional & Environmental Medicine, 99, 37–46.
Kale, M., Rathore, N., John, S., Bhatnagar, D., Nayyar, S. S., & Kothari, V. (1999). The protective effect of vitamin E in pyrethroid-induced oxidative stress in rat tissues. Journal of Nutritional and Environmental Medicine, 9, 281–287.
Acknowledgments
This work was supported by FAR from UNICAM to R.G.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhivya Vadhana, M.S., Nasuti, C. & Gabbianelli, R. Purine Bases Oxidation and Repair Following Permethrin Insecticide Treatment in Rat Heart Cells. Cardiovasc Toxicol 10, 199–207 (2010). https://doi.org/10.1007/s12012-010-9079-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-010-9079-6