Abstract
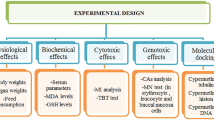
The pyrethroid imiprothrin is widely used worldwide for control of insects in the agriculture and public health sectors. No sufficient information is however available concerning detoxification gene expression, i.e., cytochrome P450 1A2 (CYP1A2) and metallothionein 1a gene, oxidative stress, lipid peroxidation, DNA damage, cytotoxicity, genotoxicity, and organ injury induced by imiprothrin in mammals. This study is designed to explain the mechanism of imiprothrin induced detoxification gene expression, DNA damage, cytotoxicity, genotoxicity, and organ toxicity in male rats. The benchmark dose (BMD) was calculated to find the best sensitive markers to imiprothrin toxicity. Imiprothrin was injected intraperitoneally (i.p.) into male rats once a day for 5 days with doses of 19, 38, and 75 mg/kg body weight (b.wt.). Imiprothrin caused a significant increase in lipid peroxidation and changes in oxidative stress biomarkers in treated rats. Significant dose-dependent changes in the liver and kidney biomarkers were observed. Histopathological alterations were seen in the liver and kidney tissue of male rats. Imiprothrin also significantly increased chromosomal aberrations (CA) and micronuclei in bone-marrow cells, and induced lipid peroxidation, oxidative stress, cytotoxicity, and liver and kidney dysfunction, and damage. Imiprothrin induced DNA damage and over detoxification gene expression of CYP1A2 and metallothionein 1a gene in hepatocytes of male rats. Imiprothrin thus shows clastogenic and genotoxic potential. The mechanism for hepatorenal toxicity and injury, genotoxicity/cytotoxicity of imiprothrin might be due to enhanced lipid peroxidation, and oxidative stress associated with overproduction of free radicals, especially reactive oxygen species, and an imbalance in redox status. From the BMD models, aspartate aminotransferase (AST), total protein, uric acid, superoxide dismutase (SOD), and micronuclei (MPEs) were very sensitive markers to imiprothrin toxicity.











Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adler ID (1984) Cytogenetic tests in mammals. In: Venitt S, Parry JM (eds) Mutagenicity Testing, A Practical Approach. IRL Press, Oxford, pp 275–306
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Al-Ahmad MM, Amir N, Dhanasekaran S, John A, Abdulrazzaq YM, Ali BR, Bastaki SM (2017) Genetic polymorphisms of cytochrome P450-1A2 (CYP1A2) among Emiratis. PLoS One 12(9):e0183424
Al-Gabri NA, Qaid MM, El-shaer NH, Ali MH, Abudabos AM (2019) Thymoquinone ameliorates pulmonary vascular damage induced byEscherichia coli–derived lipopolysaccharide via cytokine downregulation in rats. Environ Sci Pollut Res 26(18):18465–18469
Antwi FB, Reddy GV (2015) Toxicological effects of pyrethroids on non-target aquatic insects. Environ Toxicol Pharmacol 40(3):915–923
Augustyniak M, Gladysz M, Dziewięcka M (2016) The Comet assay in insects—Status, prospects and benefits for science. Mutat Res/Rev Mutat Res 767:67–76
Awad ME, Abdel-Rahman MS, Hassan SA (1998) Acrylamide toxicity in isolated rat hepatocytes, Toxicol. In Vitro 12:699–704
Bachmann K (2009) Drug Metabolism. In: Hacker M, Messer W, Bachmann K (eds) Pharmacology. Academic Press, pp 131–173
Banerjee BD, Seth V, Ahmed RS (2001) Pesticide-induced oxidative stress: perspectives and trends. Rev Environ Health 16:1–40
Barham D, Trinder P (1972) An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst 97(151):142–145
Bay BH, Jin R, Huang J, Tan PH (2006) Metallothionein as a prognostic biomarker in breast cancer. Exp Biol Med (Maywood) 231:1516–1521
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Blair A, Zahm SH (1995) Agricultural exposures and cancer. Environ Health Perspect 103:205–208
Bradberry SM, Cage SA, Proudfoot AT, Vale JA (2005) Poisoning due to pyrethroids. Toxicol Rev 24(2):93–106
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
California Environmental Protection Agency (2003). Department of Pesticide Regulation; Toxicology Data Review Summaries. Available from: http://www.cdpr.ca.gov/docs/toxsums/toxsumlist.htm on Imiprothrin as of April 21, 2003. Available at U.S. National Library of Medicine National Center for Biotechnology Information, IMIPROTHRIN, Hazardous Substances DataBank Number: 7003, Related PubChem Records: Related CIDs: 123622. https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7003. (Seen it in 5 March 2020)
CLH (2016). Imiprothrin, Proposal for harmonised classification and labelling based on regulation (EC) No 1272/2008 (CLP Regulation), Annex VI, Part 2, EC Number: 428-790-6 CAS Number: 72963-72-5 Index Number: 613-259-00-5, Version number: 2 Date: February 2016. https://echa.europa.eu/documents/10162/33091d2b-f769-e4c0-8e80-dcd666300137
Corcellas C, Feo ML, Torres JP, Malm O, Ocampo-Duque W, Eljarrat E, Barceló D (2012) Pyrethroids in human breast milk: occurrence and nursing daily intake estimation. Environ Int 47:17–22
Cortés-Iza SC, Rodríguez AI (2018) Oxidative stress and pesticide disease: a challenge for toxicology. Revista de la Facultad de Medicina 66(2):261–267
Dakeishi M, Murata K, Tamura A, Iwata T (2006) Relation between benchmark dose and no-observed-adverse-effect level in clinical research: effects of daily alcohol intake on blood pressure in Japanese salesmen. Risk Anal 26(1):115–123
El-Wakf AM (1998) Modulation of bromobenzene induced hepatotoxicity in rat by post toxicant treatment with glutathione. J Egyptian Germany Soc Zool (Comparative Physiology) 27:99–111
Emara AM, Draz EI (2007) Immunotoxicological study of one of the most common over-the-counter pyrethroid insecticide products in Egypt. Inhal Toxicol 19(12):997–1009
Fairbairn DW, Olive PL, O'Neill KL (1995a) The comet assay: a comprehensive review. Mutat Res/Rev Genet Toxicol 339(1):37–59
Fairbairn DW, Olive PL, O'Neill KL (1995b) The comet assay: a comprehensive review. Mutat Res/Rev Genet Toxicol\ 339(1):37–59
Fukai T, Ushio-Fukai M (2011) Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15(6):1583–1606
Giray B, Gürbay A, Hincal F (2001) Cypermethrin-induced oxidative stress in rat brain and liver is prevented by vitamin E or allopurinol. Toxicol Lett 118(3):139–146
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177(2):751–766
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases, the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hara M. (1992). In vitro chromosomal aberration test of S-41311 in Chinese hamster lung cells Sumitomo Chemical Co., Ltd. SGT-20-0024
Hassana E, Mohamed M (2017) Role of vitamin E in reduction of oxidative stress induced by pyrethroids on rat liver. Zagazig J Forensic Med 15(1):1–13
Hayes D, Pulford D (1995) The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol 30:445–600
Huang PC, Morris S, Dinman J, Pine R, Smith B (1987) Role of metallothionein in detoxification and tolerance to transition metals. Experientia Suppl 52:439–446
Jabłońska-Trypuć A (2017) Pesticides as inducers of oxidative stress. React Oxy Species 3:96–110
Jeffery EH (2002) Biochemical Basis of Toxicity. In: Hascher WM et al (eds) Handbook of Toxicologic Pathology, 2nd edn. Academic Press, pp 15–37
Kalender S, Kalender Y, Ogutcu A, Uzunhisarikli M, Durak D, Anikgoz F (2004) Endosulfan-induced cardiotoxicity and free radical metabolism in rats: the protective effect of vitamin E. Toxicology 202:227–235
Karami-Mohajeri S, Abdollahi M (2011) Toxic influence of organophosphate, carbamate, and organochlorine pesticides on cellular metabolism of lipids, proteins, and carbohydrates: a systematic review. Hum Exp Toxicol 30(9):1119–1140
Khan SM, Sobti RC, Kataria L (2005) Pesticide-induced alteration in mice hepato-oxidative status and protective effects of black tea extract. Clin Chim Acta 358(1-2):131–138
Kolaczinski JH, Curtis CF (2004) Chronic illness as a result of low-level exposure to synthetic pyrethroid insecticides: a review of the debate. Food Chem Toxicol 42(5):697–706
Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V (2001) Method for the measurement of antioxidant activity in human fluids. J Clin Pathol 54(5):356–361
Kosif R, Ranan GA, Aynure O (2008). The effects of oral administration of Aloe vera [barbadensis] on rat central nervous system: an experimental preliminary study. Neuroanatomy 7:22–27
Limón-Pacheco J, Gonsebatt ME (2009) The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat Res 674(1-2):137–147
Lioi MB, Scarfi MR, Santoro A, Barbieri R, Zeni O, Di Berardino D, Ursini MV (1998) Genotoxicity and oxidative stress induced by pesticide exposure in bovine lymphocyte cultures in vitro. Mutat Res/Fundament Mol Mech Mutagen 403(1-2):13–20
Mansour SA, Mossa ATH (2009) Lipid peroxidation and oxidative stress in rat erythrocytes induced by chlorpyrifos and the protective effect of zinc. Pestic Biochem Physiol 93:34–39
Mansour SA, Mossa ATH (2010) Oxidative damage, biochemical and histopathological alterations in rats exposed to chlorpyrifos and the antioxidant role of zinc. Pestic Biochem Physiol 96(1):14–23
Matsuo N (2019) Discovery and development of pyrethroid insecticides. Proceed Jpn Acad Ser B 95(7):378–400
Miles AT, Hawksworth GM, Beattie JH, Rodilla V (2000) Induction, regulation, degradation, and biological significance of mammalian metallothioneins. Crit Rev Biochem Mol Biol 35:35–70
Mirvaghefi A, Ali M, Poorbagher H (2016) Effects of vitamin C on oxidative stress parameters in rainbow trout exposed to diazinon. Su Ürünleri Dergisi 33(2):113–120
Mohafrash SMM, Abdel-Hamid HF, Mossa AH (2017) Adverse effects of sixty days sub-chronic exposure to β–cyfluthrin on male rats. J Environ Sci Technol 10(1):1–12
Moore PD, Patlolla AK, Tchounwou PB (2011) Cytogenetic evaluation of malathion-induced toxicity in Sprague-Dawley rats. Mutat Res/Genet Toxicol Environ Mutagen 725(1-2):78–82
Morgan MK (2012) Children’s exposures to pyrethroid insecticides at home: a review of data collected in published exposure measurement studies conducted in the United States. Int J Environ Res Public Health 9(8):2964–2985
Mossa ATH, Refaie AA, Ramadan A, Bouajila J (2013) Antimutagenic effect of Origanum majorana L. essential oil against prallethrin-induced genotoxic damage in rat bone marrow cells. J Med Food 16:1101–1107
Mossa AH, Heikal TM, Belaiba M, Raoelison EG, Ferhout H, Bouajila J (2015) Antioxidant activity and hepatoprotective potential of Cedrelopsis grevei on cypermethrin induced oxidative stress and liver damage in male mice. BMC Complement Altern Med 15:251–261
Mossa ATH, Rasoul MAA, Mohafrash SM (2017) Lactational exposure to abamectin induced mortality and adverse biochemical and histopathological effects in suckling pups. Environ Sci Pollut Res 24(11):10150–10165
Ncir M, Ben SG, Kamoun H, Makni AF, Khabir A, El Feki A, Saoudi M (2016) Histopathological, oxidative damage, biochemical, and genotoxicity alterations in hepatic rats exposed to deltamethrin: modulatory effects of garlic (Allium sativum). Can J Physiol Pharmacol 94(6):571–578
Nessiem AL, Bassily NS, Metwally SA (2003) Comparative histopathological evaluation of permethrin, pirimiphos methyl and bendiocarb toxicities in testes, liver and kidney of rat. Egypt J Hosp Med 11:58–73
Nishikimi M, Roa NA, Yogi K (1972) The occurrence of superoxide anionin the reaction of reduced phenazine methosulfate and molecularoxygen. Biochem Biophys Res Commun 46:849–854
Odunola OA, Gbadegesin MA, Owumi SE, Somade OT (2012) Induction of micronuclei in bone marrow cells and hepatotoxicity of one of the most common over-the-counter pyrethroid insecticide products in Nigeria. Toxicol Environ Chem 94(9):1822–1831
OECD (2016). Test No. 489: In Vivo Mammalian Alkaline Comet Assay, Sect. 6. Organisation for Economic Co-operation and Development (OECD) Publishing, Paris. Adopted: 29 July 2016
Organization for Economic Co-operation and Development (OECD) (1997): Guideline 475 Genetic toxicology: in vivo mammalian bone marrow cytogenetic test-chromosome analysis, adopted on July 21, 1997
Owumi SE, Dim UJ (2019) Manganese suppresses oxidative stress, inflammation and caspase-3 activation in rats exposed to chlorpyrifos. Toxicology Reports 6:202–209
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Poulsen CB, Borup R, Borregaard N, Nielsen FC, Møller MB, Ralfkiaer E (2006) Prognostic significance of metallothionein in B-cell lymphomas. Blood 108:3514–3519
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63
Romero A, Ares I, Ramos E, Castellano V, Martinez M, Martinezlarranaga MR, Anadon A, Martinez MA (2015) Evidence for dose-additive effects of a type II pyrethroid mixture in vitro assessment. Environ Res 138:58–66
Satoh K (1978) Serum lipid peroxide in cerebrovascular disorders deter-mined by a new colorimetric method. Clin Chim Acta 15:37–43
Schmid W (1973) Chemical mutagen testing on in vivo somatic mammalian cells. Agents Actions 3:77–85
Schmid W (1976) The Micronucleus Test for Cytogenetic Analysis. In: Hollaender A (ed) Chemical Mutagenesis, Principals and Methods for Their Detection, vol 4. Plenum Press, New York, pp 31–53
Seow A, Zhao B, Lee EJ, Poh WT, Teh M, Eng P, Wang YT, Tan WC, Lee HP (2001) Cytochrome P4501A2 (CYP1A2) activity and lung cancer risk: a preliminary study among Chinese women in Singapore. Carcinogenesis 22(4):673–677
Si M, Lang J (2018) The roles of metallothioneins in carcinogenesis. J Hematol Oncol 11(1):107
Simpkins CO (2000) Metallothionein in human disease. Cell Mol Biol 46:465–488
Taiwo VO, Nwagbara ND, Suleiman R, Angbashim JE, Zarma MJ (2008) Clinical signs and organ pathology in rats exposed to graded doses of pyrethroids-containing mosquito coil smoke and aerosolized insecticidal sprays. Afr J Biomed Res 11:97–104
Tang W, Wang D, Wang J, Wu Z, Li L, Huang M, Xu S, Yan D (2018) Pyrethroid pesticide residues in the global environment: an overview. Chemosphere 191:990–1007
Tsangaris GT, Tzortzatou-Stathopoulou F (1998) Metallothionein expression prevents apoptosis: a study with antisense phosphorothioate oligodeoxynucleotides in a human T cell line. Anticancer Res 18:2423–2434
Tucker SB, Flannigan SA (1983) Cutaneous effects from occupational exposure to fenvalerate. Arch Toxicol 54(3):195–202
United States Environmental Protection Agency (1998) Pesticide Fact Sheet: Imiprothrin. EPA Shaughnessy Code (OPP Chemical Code): 004006
Wakeling EN, Neal AP, Atchison WD (2012) Pyrethroids and their effects on ion channels. Pesticides—advances in chemical and botanical pesticides. InTech, Rijeka, pp 39–66
Wang X, Zhai W (1988) Cellular and biochemical in bronchoalveolar lavage fluids of rats exposed to fenvalerate. Zhongguo Yaolixue YuDulixue Zoghi 2:271–276
Webster NR, Nunn JF (1998) Molecular structure of free radicals and their importance in biological reactions. Br J Anaesth 60(1):98–108
Weinlich G, Eisendle K, Hassler E, Baltaci M, Fritsch PO, Zelger B (2006) Metallothionein-overexpression as a highly significant prognostic factor in melanoma: a prospective study on 1270 patients. Br J Cancer 94:835–841
Wolf P.L., Williams D., Tsudaka T., Acosta L. (1972). Methods and Techniques in Clinical Chemistry, Wiley-Interscience, a Division of Wiley, New York, London, Sydney, Toronto
Yamada T, Asano H, Miyata K, Rhomberg LR, Haseman JK, Greaves P, Greim H, Berry C, Cohen SM (2019) Toxicological evaluation of carcinogenicity of the pyrethroid imiprothrin in rats and mice. Regul Toxicol Pharmacol 105:1–14
Acknowledgements
The authors are thankful to the NRC to support this work and Prof. Dr. Wagdy Khalil, department of cell biology, NRC for helping in cytochrome P450 1A2 (CYP1A2) and metallothionein 1a gene expression and comet assay.
Author information
Authors and Affiliations
Contributions
All authors were involved in the design of the study and collection and analysis of data. EH and AM performed the genotoxic analysis and interpretation. NE performed the histopathology study and interpretation. SM and AM performed the biochemical and BMD analysis and interpretation all the data obtained from this study and was a major contributor in writing the manuscript. All authors read and approved the final manuscript
Corresponding author
Ethics declarations
Ethics approval
All experiments were completed under the standard conditions in the Animal Breeding House (ABH), of the National Research Centre (NRC), Dokki, Cairo, Egypt. The Local Ethics Committee at the National Research Centre (NRC) approved the ABH (without number).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they no conflict of interest.
Additional information
Responsible editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohafrash, S.M.M., Hassan, E.E., El-shaer, N.H. et al. Detoxification gene expression, genotoxicity, and hepatorenal damage induced by subacute exposure to the new pyrethroid, imiprothrin, in rats. Environ Sci Pollut Res 28, 33505–33521 (2021). https://doi.org/10.1007/s11356-021-13044-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13044-z