Abstract
Rainbow trout (Oncorhynchus mykiss) with a starting weight of 397.28 ± 3.21 g were fed different ratios (G1-0.00%, G2-0.010%, G3-0.025%, and G4-0.050%) of boric acid-supplemented feed for 140 days. The effects of dietary boric acid on oxidative stress parameters, growth performance, haematology and some biochemical parameters were investigated after the feeding period. The addition of boric acid to trout feed positively affected growth performance; the final weights of the groups were 928.15 ± 5.73 g, 955.87 ± 8.67 g, 994.24994,75 ± 7.46 g, and 976.80976,80 ± 6.26 g for the control group and the three experimental groups, respectively. The lowest feed conversation ratio (FCR) was 1.19 (G3) whereas the highest was 1.42 (G1). The lowest protein efficiency ratio was 1.63 (G1), while the highest was 1.95 (G3). In this study, it was observed that boric acid added to the feed changed muscle and blood oxidative stress parameters in rainbow trout, increased the growth performance of rainbow trout, and affected blood and biochemistry values.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
The aquaculture industry is one of the fastest growing food sectors worldwide and plays an important role in ensuring global food security. While aquaculture hunting has decreased in recent years, aquaculture has been increasing very rapidly. Currently, more than half of the fish consumed for human food is produced by the aquaculture sector, and this level of production is predicted to increase further [1]. Therefore, strategies are required to ensure the sustainable growth of fish farming, which is crucial both environmentally and economically [2].
Rainbow trout ranks 13th among the world's most commonly farmed fish species, and the production of rainbow trout climbed from 340 thousand tonnes in 2000 up to approximately 740 thousand tonnes in 2020 [3]. To achieve sustainability in the farming of carnivorous species like rainbow trout, it is important to reduce or eliminate the proportions of fishmeal and fish oil in their feed. Substituting vegetable protein sources for fish meal, a fundamental ingredient in the feed regimens of carnivorous animals, has a negative impact on growth performance. Further investigation is required in this field to effectively and efficiently include additional vegetable protein and oil sources into fish meals in a sustainable and productive manner.
Many studies have been carried out to increase the growth performance of fish, protect fish health, and improve meat quality [4,5,6,7,8,9,10,11,12,13].
Boron is a vital element at low levels, but it can be harmful to plants and animals when present at higher amounts [14]. Although it is not well defined how boron functions in animals, it is necessary for nutrition and physiological processes. In addition, boron plays a role in immune response, bone growth, and mineral and endocrine metabolisms [15,16,17]. It is required for embryonic development, larval stages, and growth of zebrafish (Danio rerio) and rainbow trout (Oncorhyncus mykiss) [18,19,20]. It has been reported that water-borne boron affects the serum biochemical parameters of Nile tilapia adversely but does not change hematological parameters. In addition, it has been reported that high levels of boron in the aquatic ecosystem harm fish health [21]. It is known that blood parameters and serum biochemistry parameters are affected adversely as a result of long-term and high-dose use of boron in rainbow trout feeds [22], and it also causes damage to the gills and liver [23].
There are also studies which indicate that boric acid positively affects growth performance [19] and changes muscle nutrient content [12] and the fatty acid profile [13] of rainbow trout when used in trace amounts. In addition, it is stated that the results will change when boric acid is applied in different size groups and over different periods of time [12]. In contrast to previous studies, the current investigation utilised smaller amounts of boron to examine its impact on muscle and blood oxidative stress markers. Additionally, this study is the first to test the effects of boric acid on large-sized rainbow trout.
Materials and Methods
Preparation of the Fish and Diet
The present study obtained approval from the Animal Experiments Local Committee in Adana, Turkey (No. 9–1/2021). Before the feeding trial, 108 rainbow trouts with an initial weight of 397.28 ± 3.21 g underwent a 14-day acclimation period, during which they were fed a commercial diet comprising 43% crude protein, 24% crude lipid, 3.9% crude cellulose, and 9% crude ash (Skretting Stavanger, Norway). This commercial diet was also used as the control diet for the feeding trial. Four different diets with varying levels of boric acid (G1: 0.00%, G2: 0.010%, G3: 0.025%, G4: 0.050% of boric acid) were prepared by incorporating boric acid into the feed. The selection of boric acid levels was based on previous studies [19, 24]. The fish were randomly distributed across 12 net cages (each measuring 1 m3 with a mesh size of 10 mm), with a stocking density of 9 fish per cage. Following the acclimation period, the fish were fed the experimental diets containing varying levels of boric acid along with the control diet for a duration of 140 days. Throughout both the acclimation and feeding trials, the fish were fed twice a day until apparent satiety. Daily monitoring of water quality parameters ensured that temperatures ranged between 9 and 13 °C, and dissolved oxygen levels remained above 9 mg/l throughout the experimental period.
The feed treatments were batch-prepared in 10 kg quantities. Initially, the powdered boric acid was diluted with 500 mL of water and incorporated into the feeds through spraying and impregnation. Subsequently, the feeds were washed to lubricate them, preventing the boric acid from leaching into the water. Finally, the feed batches were air-dried in the shade and stored in covered buckets. The water lubrication method was similarly applied to the control group.
Calculations of Growth-Related Performance
The specific growth rate (SGR, % day−1) is calculated as:
where w1 and w0 are the wet weights at times t1 and t0.
The feed conversion ratio (FCR) is computed as:
where Wfinal and Winitial are the live weights (g) of the fish on the initial day (t) and the final (T) day.
The protein efficiency ratio (PER) is calculated by dividing weight gain (g) by the protein intake (g) [25].
At the conclusion of the feeding trial, the fish were anesthetized using 300 ppm of 2-phenoxyethanol and promptly wiped with 70% ethanol. Blood samples were then collected from the vena caudalis using heparinized syringes. These samples were divided into standard lavender-top blood collection tubes containing anticoagulant (EDTA) for hematological analysis and standard red-top (SST™ II) advance serum separator tubes for serum biochemical parameter analysis. The latter samples underwent centrifugation at 13,000 × g for 10 min at 4 °C to obtain serum. Hematological parameters were analyzed immediately, while serum biochemical samples were stored at -80 °C until analysis.
Red blood cell (RBC), mean cell volume (MCV), mean cell hemoglobin (MCH), mean cell hemoglobin concentration (MCHC), hematocrit (Hct), and hemoglobin (Hb) were analyzed using a hematology auto analyzer MS4-S (Melet Schloesing Laboratories, Osny, France). To ensure the accuracy of the automated blood count device results, a manual hematological analysis was conducted on all blood samples immediately after collection into K3EDTA tubes, following the method outlined by Blaxhall and Daisley [26]. Serum alkaline phosphatase (ALP), glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), glucose (GLU), albumin (ALB), cholesterol (CHOL), total protein (TPR), and globulin (GLO) levels were measured using a biochemical analyzer (MScan II, Melet Schloesing, Osny, France).
Oxidative Stress Parameters
TAS levels were measured using commercially available kits from Relassay (Cat no:RL0017), Turkey [27]. Determination of Total Oxidant Status (TOS) levels were assessed using commercially available kits from Relassay (Cat no:RL0024), Turkey [28]. The ratio of TOS to TAS is accepted as the oxidative stress index (OSI. For calculation, the resulting unit of TAS is converted to μmol/L, and OSI value is calculated according to the following formula: OSI (arbitrary unit = TOS (μmol H2O2 equivalent/L / TAC (μmol Trolox equivalent/L [29,30,31]. As a product of lipid peroxidation, the level of malondialdehyde (MDA) was determined according to [32]. The measurements of erythrocyte and tissue supernatant myeloperoxidase (MPO) enzyme activity were performed according to [33]. The protein concentration of each sample was determined spectrophotometrically at 595 nm based on Bradford method [34].
Statistics
The data obtained from each treatment group were analysed using a one-way ANOVA, followed by post hoc Tukey's honestly significant difference (HSD) tests. The statistical analyses were performed using SPSS 18.0 software (Illinois, USA). There is a notable distinction when the p-value is < 0.05.
Results
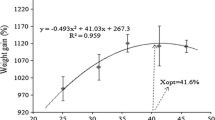
The present study calculated the growth performance of a portion-size rainbow trout fed with the feeds containing boric acid at different rates for 140 days. The results are presented in Table 1. The study revealed that the inclusion of boric acid in the diet positively impacted the growth performance of rainbow trout. Moreover, the observed differences in growth performance among the experimental groups were found to be statistically significant at a significance level of P < 0.05. The rainbow trouts in the four experiment groups with an average initial weight of 397.28 g reached the final weights of 928.15 ± 5.73 g, 955.24 ± 8.67 g, 994.75 ± 7.46 g, and 976.80 ± 6.26 g, respectively, and the highest live weight gain (597.48 ± 7.46 g) was achieved in the 3rd group which was fed with 0.025% boric acid.
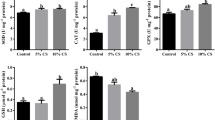
In the present study, oxidative stress parameters were determined from the blood serum and muscle tissue samples of the fish, and the results are presented in Table 2. The sera were obtained from the blood samples taken from the fish, and the amounts of TAS, TOS, OSI, MPO, and MDA as a result of the study were determined in the blood sera. TAS, TOS, MPO, MDA, and MP in the muscle tissue were determined.
TAS is 1.14 (mmol/L) in the control group. The boric acid which was added to the diet is found to change TAS levels of rainbow trout, and it varies between 1.72 and 1.40 (mmol/L) in the treatment groups. The boric acid decreased the Total Oxidant Status (TOS) of the rainbow trout, and there is a statistical difference between the study groups and the control group (P < 0.05). The results were calculated as 5.30 ± 0.33, 10.90 ± 0.23, 11.35 ± 0.62, and 11.35 ± 0.62 µmol/L, for G1, G2, G3, and G4, respectively. In addition, boric acid feed caused an increase in Malondialdehyde level and a decrease in Myeloperoxidase level in the blood serum. The analyses of the muscle samples taken as a result of the research concluded that boric acid added feed caused an increase in TAS and MP levels of rainbow trout, while it caused a decrease in TOS, MPO, and MDA levels.
At the end of the feeding period, blood samples were taken from the rainbow trout which were fed with different rates of boric acid-supplemented feed for 140 days. The results of the hematology and serum biochemistry parameters are presented in Tables 3 and 4.
Discussion
The two most common measures of responses in fish to a particular diet or component are growth and feed use. Growth is measured in the function of specific nutrient gain (e.g., protein), weight gain, or length. One goal of aquaculture is to maximize production in terms of biomass. Therefore, body weight gain is a common performance measure [35]. [12, 13] had positive results in their feeding study with boric acid but emphasized the necessity of performing feeding trials with different size groups and doses. They could not foresee what results would be obtained in long-term feeding studies [12, 13]. In the present study, lower doses of boron were used compared to previous feeding studies, and five-time feeding was applied for a relatively long time. In addition, nutritive elements are usually investigated in smaller fish. The present study used rainbow trout of portion size, and very promising results were obtained regarding growth performance.
Many active substances are tested to increase the growth performance of fish. Öz and Dikel reported that garlic increases the growth performance in their 2022 study with garlic-supplemented feed [36]. In a study conducted by Öz et al. [37], it was observed that the administration of black cumin oil results in enhanced growth performance and improved immune system functionality in nile tilapia. It was reported in another study that excess glycine and glutamate in the rainbow trout diet improves the digestibility of proteins, lipids, and most of the amino acids and fatty acids, which is positively reflected in their growth performance [38]. The second factor that indicates performance in feeding trials is feed use. It refers to the extent to which the organism's food is converted to growth, which is especially important when comparing the economic cost and potential of feeds to contaminate the culture medium [35]. The feed utilisation of the research groups was determined as 755.50 ± 4.55 g, 732.32 ± 3.17 g, 711.57 ± 3.42 g, and 734.11 ± 2.35 g. The present study revealed that the group which received the feed supplementation of 0.025% boric acid exhibited the lowest feed conversion ratio (FCR) as 1.19. Conversely, the control group demonstrated the highest FCR ratio as 1.42.
A combination of dietary cinnamon and a probiotic has been reported to improve immunity, digestive enzyme activity, and growth performance of rainbow trout under stress from stockpiles. While the highest FCR in the aforementioned study was calculated in the control group (1.53), it was reduced to 1.25 in the treatment groups [39]. Similarly in the present study, the fish which were fed with boric acid supplementation demonstrated higher final weight, weight gain, and lower food conversation ratio than the individuals which were fed non-supplemented feed, indicating that boric acid in the diet exerts a growth-promoting effect. Consistent with these research findings, some studies also report the effects of nutritional supplements on fish growth. Naderi et al. [40] reported that dietary vitamin E contributes to the growth performance of rainbow trout. Additionally, Dikel et al. found in [41] that L-carnitine addition to rainbow trout feeds affects growth performance positively.
TAS in fish indicates the overall antioxidant capacity and protection against oxidative stress in their tissues caused by free radicals and reactive oxygen species [42]. TAS assessment is crucial to evaluate the protective effects of antioxidants against oxidative stress and to understand the impact of oxidative stress on fish physiology [43]. Moreover, TAS has been used to assess the effects of dietary supplementation on some fish such as the evaluation of the effects of different dietary vitamin E levels on growth performance, non-specific immune responses, and disease resistance in parrot fish [44]. The addition of compounds or chemicals to fish diets can significantly impact Total Antioxidant Status (TAS) of fish. For instance, dietary inclusion of astaxanthin has been found to increase TAS in fish, indicating its strong antioxidant properties [45]. On the other hand, the addition of Oregano vulgare extract to fish diets has been shown to decrease the activity of antioxidant enzymes and total antioxidant capacity [46]. Conversely, the inclusion of betaine in plant-protein-based diets has been found to enhance the antioxidant status in fish [47]. Consequently, additives to fish diets may affect their Total Antioxidant Status (TAS) differently. While compounds such as astaxanthin can boost TAS, others such as Oregano vulgare extract may reduce it. The type and quantity of fish oil, along with the presence of additives such as tannic acid and betaine, also influence TAS. Additionally, the composition of the basal diet impacts the fish's antioxidant status significantly. In the present study, significant increases in TAS levels are observed in G2 and G3 (1.72 mmol/L) compared to the control group (G1), while this increase was slightly lower (1.40 mmol/L) in G4. It indicates that the feeds with boric acid can enhance antioxidant capacity up to a certain dosage. TAS levels in muscle tissue observed a slight increase in G2 and G3, with a more pronounced increase in G4 (10.45 μmol/mg protein). It suggests that the feeds which contain boric acid may improve antioxidant capacity in muscle tissue.
Total Oxidant Status (TOS) in fish refers to the measurement of the different oxidant species present in the biological samples, providing valuable insights into the oxidative status and the presence of oxidant compounds. In conjunction with Total Antioxidant Capacity (TAC), TOS has been proposed as a valuable endpoint to assess the oxidative status in fish after exposure to chemicals, reflecting the combined action of different oxidants present in the biological sample [48]. Furthermore, TOS has been utilized in various studies to assess the oxidative stress and redox balance in fish, which poses significance in understanding the impact of oxidative stress on fish physiology [49, 50]. For instance, the inclusion of Oregano vulgare extract in fish diets has been found to decrease the activity of antioxidant enzymes and total antioxidant capacity, thereby affecting TOS [46]. Oxidative Stress Index (OSI) in fish serves as a comprehensive indicator of the degree of oxidative stress, providing valuable insights into the balance between total oxidant status (TOS) and total antioxidant capacity (TAC). OSI is calculated as the ratio of TOS to TAC, making it a reliable measure of the oxidative status in fish. The addition of compounds or chemicals to fish diet can have a significant impact on their Oxidative Stress Index (OSI). The use of various dietary carbohydrate and lipid sources has been found to affect the oxidative status, as evidenced by changes in antioxidant enzyme activities, lipid peroxidation, and OSI in European sea bass juveniles [51]. Furthermore, the quality of dietary oils has been demonstrated to influence OSI in Atlantic cod, and various dietary oil qualities affect oxidative stress as indicated by changes in lipid peroxidation and antioxidant enzyme activities [52]. In the blood samples in the present study, the values of TOS and OSI were elevated in G2, G3, and G4 compared to G1. This increase suggests that the feeds containing boric acid may increase oxidative stress.
Myeloperoxidase (MPO) is an enzyme found in neutrophils and a marker of neutrophil activation and inflammation. It is involved in the production of hypochlorous acid, a potent antimicrobial agent. In fish, MPO activity has been detected in various tissues and is used as an indicator of neutrophil activation and degranulation [53]. Malondialdehyde (MDA) is a naturally occurring product of lipid peroxidation and commonly used as an indicator of oxidative stress and lipid peroxidation in fish. It is considered as a basic compound of cellular damage by toxins, and it is a biomarker of oxidative stress occurring in cell components [54]. Microprotein (MP) is a term used to refer to small proteins or peptides. The addition of compounds or chemicals to fish diet may have a significant impact on various biomarkers, including Myeloperoxidase (MPO), Malondialdehyde (MDA), and Microprotein (MP). For instance, the inclusion of dietary probiotic bacteria and processed yeast has been shown to influence MPO activity, which plays a role in regulating reactive oxygen species (ROS) and killing invading pathogens through the production of hypochlorous acid and strong oxidants [55]. Furthermore, the addition of plant essential oils as fish diet additives has been reported to enhance fish health and stability in feed, potentially impacting MDA and overall health status [56]. In the present study, the levels of MPO in blood samples decreased significantly in G2 and G3 but were reduced less in G4. MDA levels increased across all treatment groups, indicating an increase in lipid peroxidation. In muscle tissue, both TOS and MPO levels decreased in all treatment groups compared to G1. It indicates that feeds with boric acid may reduce oxidative stress and inflammation in muscle tissue. The levels of MDA decreased in all treatment groups compared to G1, while MP levels increased in G2 and G4 groups. It shows that feeds which contain boric acid may reduce lipid peroxidation and preserve protein structure in muscle tissue. The present study demonstrates that the feeds which are supplemented with boric acid may affect oxidative stress parameters in the blood and muscle tissues of rainbow trouts in various ways. Boric acid at certain doses can enhance antioxidant capacity and also increase oxidative stress and lipid peroxidation. These findings highlight the importance of further research on the potential benefits and risks of boric acid.
Haematology is a significant method to assess the health of fish in connection to several factors such as diseases, stress, nutrition, and alterations in environmental circumstances [57,58,59]. Dietary boric acid decreased the red blood cell (RBC) value of the rainbow trout. It was found to be 1.77 ± 0.07, 1.50 ± 0.04, 1.31 ± 0.0, and 1.29 ± 0.05 for the three experiment groups. In their study, Fazio et al. observed RBC values ranging between 1.55–4.55 in the blood samples from the trout farms in Italy and Turkey, and the present results are close to those obtained from the rainbow trout in Turkey [60]. According to the present research findings, boric acid has the ability to impact hematopoietic tissue, resulting in a reduction in the release of red blood cells (RBCs) into the circulatory system. Based on the findings presented by Joshp et al. [61], the experimental groups exhibited a notable reduction in red blood cell (RBC) count compared to the control group. This decrease may result from the inhibition of erythropoiesis and an elevated rate of erythrocyte breakdown in hematopoietic organs. In fish, RBCs absorb oxygen in the gills and release it into the tissues. A decrease in RBC below normal limits causes insufficient oxygen to be carried to the tissues. In the current study, although boric acid reduced the RBC value of rainbow trout compared to the control group, RBC remained within normal limits. Boric acid may adversely affect the health of fish in higher doses and long-term use.
The hematocrit (Hct) and haemoglobin (Hb) levels of the fish displayed a slight initial rise which was followed by a subsequent decline in response to the increasing concentration of boric acid. The groups that were fed boric acid-supplemented feed exhibited higher values of Mean cell volume (MCV), Mean cell haemoglobin concentration (MCHC), and Mean cell haemoglobin (MCH) in comparison to the control group. However, no difference was determined among the treatment groups. The hematological parameters of the present study are in line with the previous studies with Oncorhynchus mykiss and Salmo trutta macrostigma species [49, 50, 60].
It was determined that boric acid addition to fish feed at increasing rates caused an increase in Alkaline phosphatase (ALP) activity compared to the control group. When the boron-applied groups are compared with each other and the control group, the results are significantly different (p < 0.05). Fasting blood Glc measurement is important in determining the health status of living things and in the follow-up of diseases. Glc level is important in the follow-up of the health status of many vital tissues, especially the liver [62]. The main source of Glc is foods of exogenous origin. However, fish use Glc produced in gluconeogenesis and glycogenolysis metabolic pathways with hormonal control unlike other creatures since they cannot directly use dietary carbohydrates [63]. Blood Glc levels (hyper- and hypo-glycemia) are a sensitive and reliable indicator of physiological response to nutrients and/or toxic pollutants that cause environmental stress in fish [64]. As the principal energy substrate, blood glucose plays a pivotal part in the metabolic processes of fish. According to Heath [65], the analysis of blood glucose levels in fish can provide valuable insights on various aspects such as their metabolic status, rate of generation and consumption, and energy requirements.
The biochemical and pathophysiological characteristics of albumin and globulins play a crucial role in the assessment of health status, disease diagnosis, and therapy monitoring. These proteins are integral components of the total protein panel. Evaluation of protein content is used as a good diagnostic tool to determine the physiological state of cells [62]. The average values of some commonly used biochemical parameters in various healthy fish species were analysed in a review study published in 2007 which reported total protein as 3.49 ± 1.007 (0.10–7.50) g/dL, albumin as 1.23 ± 0.639 (0.10–3.20) g/dL, globulin as 2.38 ± 0.664 (0.40 -4.37) g/dL, urea as 5.33 ± 4.056 (0.00–18.00) mg/dL, cholesterol from blood lipids as 248.62 ± 146.248 (0.10–714.29) mg/dL, and triglycerides as 225.90 ± 175.995 mg/dL [66]. In the present study, boric acid which was supplemented to fish feed changed the biochemical values of blood samples. Likewise, previous studies obtained similar results [21,22,23,24, 67, 68].
Conclusion
In the current research, different proportions of boric acid were added to portion-size rainbow trout feed, and the fish were fed for 140 days. Boric acid added to the feed at the end of the feeding period positively affected the growth performance of large rainbow trout. It did not have a negative effect on blood hematology and biochemistry parameters. These findings highlight the potential advantages of using boric acid as a supplementary substance in the diet of rainbow trout, since it has been shown to promote their growth while maintaining their haematological and biochemical well-being. Further studies are needed to determine the optimal application frequency of substances such as boric acid and to evaluate their effects on the immune system and infection with parasites or other diseases.
Data Availability
No datasets were generated or analysed during the current study.
Change history
01 July 2024
The original version of this article was updated. The ORCID has been updated.
References
FAO. 2023. World Food and Agriculture – Statistical Yearbook 2023. Rome. https://doi.org/10.4060/cc8166en
Rodgers EM, Gomez Isaza DF (2024) The growth-promoting effects of exercise in finfish: A systematic review and meta-analysis. Rev Aquac 16(2):942–953
FAO. 2022. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation. Rome, FAO. https://doi.org/10.4060/cc0461en
Acar Ü, Kesbiç OS, Yılmaz S, İnanan BE, Zemheri-Navruz F, Terzi F, Parrino V (2021) Effects of essential oil derived from the bitter orange (Citrus aurantium) on growth performance, histology and gene expression levels in common carp juveniles (Cyprinus carpio). Animals 11(5):1431
Adineh H, Harsij M, Jafaryan H, Asadi M (2020) The effects of microencapsulated garlic (Allium sativum) extract on growth performance, body composition, immune response and antioxidant status of rainbow trout (Oncorhynchus mykiss) juveniles. J Appl Anim Res 48(1):372–378
Ashour, M., Mabrouk, M. M., Abo-Taleb, H. A., Sharawy, Z. Z., Ayoub, H. F., Van Doan, H., ... & Goda, A. M. A. (2021). A liquid seaweed extract (TAM®) improves aqueous rearing environment, diversity of zooplankton community, whilst enhancing growth and immune response of Nile tilapia, Oreochromis niloticus, challenged by Aeromonas hydrophila. Aquaculture, 543:736915
Ashour, M., Mabrouk, M. M., Ayoub, H. F., El-Feky, M. M., Zaki, S. Z., Hoseinifar, S. H., ... & Goda, A. M. S. (2020). Effect of dietary seaweed extract supplementation on growth, feed utilization, hematological indices, and non-specific immunity of Nile Tilapia, Oreochromis niloticus challenged with Aeromonas hydrophila. Journal of Applied Phycology, 32:3467–3479
Inanan BE, Acar Ü (2019) Evaluation of sugar beet leave extracts in goldfish (Carassius auratus) diets: Effects on blood and semen parameters. Acta Aquatica Turcica 15(4):458–468
Inanan BE, Acar Ü, İnanan T (2021) Effects of dietary Ferula elaeochytris root powder concentrations on haematology, serum biochemical parameters, spermatozoa parameters, and oxidative status in tissues of males goldfish (Carassius auratus). Aquaculture 544:737087
Öz M (2018) Effects of garlic (Allium sativum) supplemented fish diet on sensory, chemical and microbiological properties of rainbow trout during storage at− 18 C. LWT 92:155–160
Öz M (2017) Çörek otu (Nigella sativa) yağının gökkuşağı alabalığının (Oncorhynchus mykiss) karaciğer yağ asidi profiline etkisi. Etlik Veteriner Mikrobiyoloji Dergisi 28(1):55–59
Öz M, Tatil T, Dikel S (2021) Effects of boric acid on the growth performance and nutritional content of rainbow trout (Oncorhynchus mykiss). Chemosphere 272:129895
Öz M, Inanan B, Dikel S (2021) Yem Kaynaklı Borun Gökkuşağı Alabalığı (Oncorhynchus mykiss) Yağ Asidi Profiline Etkisi. Avrupa Bilim ve Teknoloji Dergisi 31:188–192
Goldbach, H. E., Huang, L., & Wimmer, M. A. (2007). Boron functions in plants and animals: recent advances in boron research and open questions. In Advances in plant and animal boron nutrition: Proceedings of the 3rd International Symposium on all Aspects of Plant and Animal Boron Nutrition (pp. 3–25). Springer Netherlands
Nielsen FH (1997) Boron in human and animal nutrition. Plant Soil 193:199–208
Kabu M, Akosman MS (2013) Biological effects of boron. Rev Environ Contam Toxicol 225:57–75
Hunt CD (1994) The biochemical effects of physiologic amounts of dietaryboron in animal nutrition models. Environ Health Perspect 102(7):35–43
Loewengart G (2001) Toxicity of Boron To Raınbow Trout: A Weight Of The-Evidence Assessment. Environ Toxicol Chem 20(4):796–803
Öz M, Inanan BE, Dikel S (2018) Effect of boric acid in rainbow trout (Oncorhynchus mykiss) growth performance. J Appl Anim Res 46(1):990–993
Rowe RI, Eckhert CD (1999) Boron is required in zebrafish. J Exp Biol 202:1649–1654
Acar Ü, İnanan BE, Zemheri F, Kesbiç OS, Yılmaz S (2018) Acute exposure to boron in Nile tilapia (Oreochromis niloticus): Median-lethal concentration (LC50), blood parameters, DNA fragmentation of blood and sperm cells. Chemosphere 213:345–350
Öz M, Karaşahin T, Aksoy NH, İnanan BE (2020) Harmful effects of dietary supplementation of boron on blood parameters of Rainbow Trout (Oncorhynchus mykiss). J Hellenıc Vet Med Soc 71(2):2227–2234
Öz M, Yavuz O, Bolukbas F (2020) Histopathology changes in the rainbow trout (Onchorhyncus mykiss) consuming boric acid supplemented fish fodder. J Trace Elem Med Biol 62:126581
Ardó L, Yin G, Xu P, Váradi L, Szigeti G, Jeney Z, Jeney G (2008) Chinese herbs (Astragalus membranaceus and Lonicera japonica) and boron enhance the non-specific immune response of Nile tilapia (Oreochromis niloticus) and resistance against Aeromonas hydrophila. Aquaculture 275(1–4):26–33
Skalli A, Robin JH (2004) Requirement of n-3 long chain polyunsaturated fatty acids for European sea bass (Dicentrarchus labrax) juveniles: growth and fatty acid composition. Aquaculture 240:399–415. https://doi.org/10.1016/j.aquaculture.2004.06.036
Blaxhall PC, Daisley KW (1973) Routine haematological methods for use with fish blood. J Fish Biol 5(6):771–781
Erel O (2004) A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem 37(4):277–285
Erel O (2005) A new automated colorimetric method for measuring total oxidant status. Clin Biochem 38(12):1103–1111
Harma M, Harma M, Erel O (2003) Increased oxidative stress in patients with hydatidiform mole. Swiss Med Wkly 133:563–536
Kosecik M, Erel O, Sevinc E, Selek S (2005) Increased oxidative stress in children exposed to passive smoking. Int J Cardiol 100:61–64
Yumru M, Savas HA, Kalenderoglu A, Bulut M, Celik H, Erel O (2009) Oxidative imbalance in bipolar disorder subtypes: a comparative study. Prog Neuropsychopharmacol Biol Psychiatry 33(6):1070–1074
Alak, G., Özgeriş, F. B., Yeltekin, A. Ç., Parlak, V., Ucar, A., Caglar, O., ... & Atamanalp, M. (2020). Hematological and hepatic effects of ulexite in zebrafish. Environmental Toxicology and Pharmacology, 80:103496
Bradley JR, Johnson DR, Pober JS (1993) Endothelial activation by hydrogen peroxide. Selective increases of intercellular adhesion molecule-1 and major histocompatibility complex class I. Am J Pathol 142(5):1598
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Lazo, J. P., & Davis, D. A. (2000). Ingredient and feed evaluation. Encyclopedia of Aquaculture, 453–463
Öz M, Dikel S (2022) Effect of garlic (Allium sativum)-supplemented diet on growth performance, body composition and fatty acid profile of rainbow trout (Oncorhynchus mykiss). Cell Mol Biol (Noisy-le-grand) 68(1):217–225
Öz, M., Üstüner, E., & Bölükbaş, F. (2023). Effects of dietary black cumin (Nigella sativa L.) oil on growth performance, hemato‐biochemical and histopathology of cypermethrin‐intoxicated Nile tilapia (Oreochromis niloticus). J World Aquac Soc. https://doi.org/10.1111/jwas.13005
Belghit I, Philip AJP, Maas RM, Lock EJ, Eding EH, Espe M, Schrama JW (2023) Impact of dietary glutamate and glycine on growth and nutrient utilization in rainbow trout (Oncorhynchus mykiss). Aquaculture 568:739311
Jasim, S. A., Hafsan, H., Saleem, H. D., Kandeel, M., Khudhair, F., Yasin, G., ... & Dadras, M. (2022). The synergistic effects of the probiotic (Lactobacillus fermentum) and cinnamon, Cinnamomum sp. powder on growth performance, intestinal microbiota, immunity, antioxidant defence and resistance to Yersinia ruckeri infection in the rainbow trout (Oncorhynchus mykiss) under high rearing density. Aquaculture Res 53(17):5957–5970
Naderi M, Keyvanshokooh S, Ghaedi A, Salati AP (2019) Interactive effects of dietary Nano selenium and vitamin E on growth, haematology, innate immune responses, antioxidant status and muscle composition of rainbow trout under high rearing density. Aquac Nutr 25(5):1156–1168
Dikel, S., Ünalan, B., Eroldoğan, O. T., & Hunt, A. Ö. (2010). Effects of dietary L-carnitine supplementation on growth, muscle fatty acid composition and economic profit of rainbow trout (Oncorhynchus mykiss). Turkish J Fisheries Aquatic Sci 10(2)
Kurhaluk, N., & Tkachenko, H. (2021). Antioxidants, lysosomes and elements status during the life cycle of sea trout Salmo trutta m. trutta L. Sci Rep 11(1):5545
Selvi M, Çavaş T, Cağlan Karasu Benli A, Koçak Memmi B, Çinkılıç N, Dincel AS, ..., Erkoc F (2013) Sublethal toxicity of esbiothrin relationship with total antioxidant status and in vivo genotoxicity assessment in fish (Cyprinus carpio L., 1758) using the micronucleus test and comet assay. Environ Toxicol 28(11):644–651
Galaz GB, Kim SS, Lee KJ (2010) Effects of different dietary vitamin E levels on growth performance, non-specific immune responses, and disease resistance against Vibrio anguillarum in parrot fish (Oplegnathus fasciatus). Asian Australas J Anim Sci 23(7):916–923
Xie JJ, Chen X, Liu YJ, Tian LX, Xie SW, Niu J (2017) Effects of dietary astaxanthin on growth performance, hepatic antioxidative activity, hsp70, and hif-1α gene expression of juvenile golden pompano (Trachinotus ovatus). Israeli J Aquac 1430:1–12
Alagawany M, Farag MR, Salah AS, Mahmoud MA (2020) The role of oregano herb and its derivatives as immunomodulators in fish. Rev Aquac 12(4):2481–2492
Mohseni M, Saltanat NL, Rastravan ME, Golalipour Y (2021) Effects of betaine supplementation in plant-protein-based diets on growth performance, haemato-immunological parameters, antioxidant status and digestive enzyme activities of juvenile Caspian trout (Salmo trutta, Kessler, 1877). Aquac Nutr 27(6):2132–2141
Teles, M., Oliveira, M., Jerez-Cepa, I., Franco-Martínez, L., Tvarijonaviciute, A., Tort, L., & Mancera, J. M. (2019). Transport and recovery of gilthead sea bream (Sparus aurata L.) sedated with clove oil and MS222: Effects on oxidative stress status. Front Physiol 10:523.
Fazio, F., Arfuso, F., Fortino, G., Piccione, G., & Faggio, C. (2015). Blood and Biometric Data on an Established Salmo trutta macrostigma (Dumeril, 1858) Population in an Italian Stream: Preliminary Results. Pakistan Journal of Zoology 47(2).
Fazio, F., Piccione, G., Saoca, C., Caputo, A. R., & Cecchini, S. (2015). Assessment of oxidative stress in Flathead mullet (Mugil cephalus) and Gilthead sea bream (Sparus aurata). Veterinární Medicína 60(12)
Castro, C., Peréz-Jiménez, A., Coutinho, F., Díaz-Rosales, P., dos Reis Serra, C. A., Panserat, S., ... & Oliva-Teles, A. (2015). Dietary carbohydrate and lipid sources affect differently the oxidative status of European sea bass (Dicentrarchus labrax) juveniles. British Journal of Nutrition, 114(10):1584–1593
Kjær MA, Aursnes IA, Berge GM, Sørensen M, Marchenko Y, Gjøen T, Ruyter B (2014) The influence of different dietary oil qualities on growth rate, feed utilization and oxidative stress in Atlantic cod. Aquac Nutr 20(2):192–204
Palić D, Andreasen CB, Menzel BW, Roth JA (2005) A rapid, direct assay to measure degranulation of primary granules in neutrophils from kidney of fathead minnow (Pimephales promelas Rafinesque, 1820). Fish Shellfish Immunol 19(3):217–227
Taysı MR, Kırıcı M, Kırıcı M, Ulusal H, Söğüt B, Taysı S (2021) The role of nitrosative and oxidative stress in rainbow trout (Oncorhynchus mykiss) liver tissue applied mercury chloride (HgCl 2). Ege Journal of Fisheries & Aquatic Sciences (EgeJFAS)/Su Ürünleri Dergisi 38(3)
Valadez-Cosmes P, Raftopoulou S, Mihalic ZN, Marsche G, Kargl J (2022) Myeloperoxidase: Growing importance in cancer pathogenesis and potential drug target. Pharmacol Ther 236:108052
Sutili FJ, Gatlin DM III, Heinzmann BM, Baldisserotto B (2018) Plant essential oils as fish diet additives: benefits on fish health and stability in feed. Rev Aquac 10(3):716–726
Fazio F (2019) Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture 500:237–242
Sula E, Aliko V, Pagano M, Faggio C (2020) Digital light micros-copy as a tool in toxicological evaluation of fish erythrocyte mor-phological abnormalities. Microsc Res Tech 83(4):362–369
Ghafarifarsani H, Hoseinifar SH, Adorian TJ, Ferrigolo FRG, Raissy M, Van Doan H (2021) The effects of combined inclu-sion of Malvae sylvestris, Origanum vulgare, and Allium hirtifolium Boiss for common carp (Cyprinus carpio) diet: Growth perfor-mance, antioxidant defense, and immunological parameters. Fish Shellfish Immunol 119:670–677
Fazio F, Saoca C, Piccione G, Kesbiç OS, Acar Ü (2016) Comparative study of some hematological and biochemical parameters of Italian and Turkish farmed rainbow trout Oncorhynchus mykiss (Walbaum, 1792). Turk J Fish Aquat Sci 16(3):715–721
Joshp PK, Bose M, Harish D (2002) Changes in certain hematological parameters in a siluroid cat fsh Clarias batrachus (Linn) exposed to cadmium chloride. Pollution Res 21(2):129–131
Kaneko JJ, Harvey JW, Bruss ML (2008) Clinical Biochemistry of Domestic Animals, 6th edn. Academic Press, San Diego
Kamalam BS, Medale F, Panserat S (2017) Utilisation of dietary carbohydrates in farmed fishes: New insights on influencing factors, biological limitations and future strategies. Aquaculture 467:3–27
Gül S, Belge-Kurutas E, Yildiz E, Sahan A, Doran F (2004) Pollution correlated modifications of liver antioxidant systems and histopathology of fish (Cyprinidae) living in Seyhan Dam Lake. Turkey Enviro Int 30:605–609
Heath AG (1995) Water pollution and fsh physiology. CRC Press, Boca Raton
Çelik E.Ş ve Bilgin S. (2007). Bazı Balık Türleri İçin Kan Protein ve Lipidlerinin Standardizasyonu. Erciyes Üniversitesi Fen Bilimleri Enstitüsü Dergisi 23(1-2):215–229
Alak G, Parlak V, Aslan ME, Ucar A, Atamanalp M, Turkez H (2019) Borax supplementation alleviates hematotoxicity and DNA damage in rainbow trout (Oncorhynchus mykiss) exposed to copper. Biol Trace Elem Res 187:536–542
Alak G, Turkez H, Parlak V, Uçar A, Özgeriş F, Yeltekin AÇ, Atamanalp M (2022) The mitigation role of borax in aluminum hydroxide-induced toxicity: A systematic research on fish modelling. Authorea. https://doi.org/10.22541/au.166973478.88343165/v1
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Mustafa ÖZ: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Validation; Visualization; review & editing Writing- Original draft preparation.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Öz, M. Effects of Boric Acid on Oxidative Stress Parameters, Growth Performance and Blood Parameters of Rainbow Trout (Oncorhynchus Mykiss). Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04276-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04276-4