Abstract
The extensive utilization of boric acid, particularly in industrial and agricultural sectors, also engenders concerns regarding the toxicity of boron and its derivatives. Particularly, the behavior of boric acid at increasing concentrations in aquatic ecosystems remains poorly understood. In light of these concerns, this study aimed to investigate the toxicity of boric acid in bivalves, which occupy a critical position in the food chain. Specimens of Ruditapes decussatus, which had not been previously exposed to any pollutants and were cultivated under controlled conditions, were subjected to three different concentrations of boric acid (0.05 mg/L, 0.5 mg/L, and 5 mg/L) in vitro for 96 h. Following the exposure period, the specimens were assessed for histological changes (the mantle, gill, and digestive gland) and specific oxidative parameters (the gill and digestive gland), including superoxide dismutase (SOD), catalase (CAT), glutathione-S-transferase, and lipid peroxidation (LPO). The research findings indicated that boric acid primarily induced oxidative damage at the applied concentrations and increased antioxidant levels (p < 0.05). Moreover, although no significant histopathological abnormalities were observed in the examined histological sections, subtle changes were noted. This study evaluated the potential adverse effects of boric acid on bivalves, which are crucial components of the aquatic food chain, utilizing histological and specific physiological parameters following its introduction into aquatic environments. It is anticipated that the findings of this study will contribute to the development of new insights and perspectives regarding the extensive use of boric acid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introductıon
Boron is recognized as an indispensable micronutrient, serving as a trace element vital for the physiological development and growth of organisms [1,2,3]. It occurs naturally in the environment, forming compounds with other elements, some of which hold considerable commercial significance [4]. For instance, boric acid, identified as a weak monobasic Lewis acid of boron [5], is of notable industrial importance. Its essentiality for plant growth has been long established [6, 7], with uptake occurring through plant consumption and, via water sources, as inorganic boron, subsequently transferring to animal species and humans [8]. Upon its discovery, boron’s significance in plant nutrition was promptly recognized, leading to its widespread application in agricultural practices [3]. The nutritional role of boron in human and animal metabolism gained elucidation in the 1980s [9,10,11]. In the European Union, boric acid finds authorization as a food additive and preservative in select food items (e.g., caviar) [10], while its utilization in animal nutrition has also been notable in recent years [4]. Boron exerts multifaceted effects on cellular signaling pathways and participates in the formation and modulation of entities involved in numerous biochemical processes. It assumes pivotal roles in the life cycle of highly organized organisms and contributes to various biological phenomena such as cellular structural integrity and enzymatic activities [12]. Furthermore, boron has been implicated in cellular signaling mechanisms, impacting the functionality of diverse organs, including the brain, while also actively modulating immune responses [7, 13, 14]. Beyond its nutritional significance, boron finds extensive utility across diverse domains.
In addition to its utilization in cosmetic, ceramic, and glass industries, boron is frequently favored within industrial sectors such as nuclear technology, materials engineering, and energy production [15]. Consequently, experimental inquiries were launched to explore its impact on clinical health subsequent to the elucidation of its biological significance and role in animal and human metabolic processes [4, 16]. As the usage of boron proliferated, inquiries emerged regarding its potential toxicological implications, juxtaposed against its protective effects on living organisms [17, 18]. The introduction of boron into air, water, or soil ecosystems can be construed as a corollary of its escalating utilization, both naturally and anthropogenically [19, 20]. This surge in boron usage has prompted scrutiny into the toxic ramifications of boric acid [21, 22]. While low concentrations of boron are generally associated with minimal toxicity in soil, water, and living organisms, investigations have delineated its adverse effects at elevated concentrations, leading to its classification within the chemical pesticide group since as early as 1948 [5]. Despite the extensive historical use of boric acid across diverse applications, from medicinal to pesticidal and industrial realms, information pertaining to its potential toxicological effects remains somewhat limited [3]. Although the available literature regarding the toxic effects of boron on animals is currently constrained, prevailing studies predominantly focus on human populations [23, 24] and rodent models [25,26,27,28]. Over the past two decades, there has been a growing awareness regarding the acute toxicity of boron on aquatic organisms, stemming from its ingress into aquatic ecosystems via both natural processes and human activities [12]. It is postulated that boron and its derivatives, particularly those introduced into freshwater bodies through agricultural and irrigation wastewater, have the potential to translocate to inland waters and subsequently to marine environments, potentially exerting toxic effects at specific concentration thresholds [29, 30]. The assessment of boron’s potential toxic effects has predominantly focused on fish species [4, 7, 12, 31,32,33,34,35,36,37,38], with limited investigations involving macroinvertebrates [3]. A common consensus derived from these studies is that boron and its derivatives possess the capacity to perturb hormone and lipid metabolism, as well as modulate the activity of numerous enzymes [34, 39, 40]. While the precise extent of these effects on biochemical processes remains incompletely elucidated [33, 34, 41], it is established that boron and its derivatives do not undergo metabolic transformations, with borates introduced into aquatic environments primarily forming boric acid and borate anions [12, 42].
As with any pollutant infiltrating aquatic ecosystems, boric acid harbors the potential to instigate oxidative stress within aquatic organisms via mechanisms involving free radicals and reactive oxygen species (ROS). Aquatic organisms, particularly bivalves, possess the capacity to mount responses to environmental pollutants through a spectrum of immune and antioxidant defense mechanisms [43,44,45]. Ruditapes species, prominent constituents of coastal ecosystems, bear substantial economic and ecological significance. Their propensity, akin to other bivalves, for pollutant accumulation through filter-feeding renders them valuable focal points in biomonitoring endeavors [46, 47]. The primary objective of this study is to elucidate the deleterious effects of boric acid, a commonly employed substance, on bivalves within aquatic environments. To this end, histopathological alterations and antioxidant responses were evaluated in the digestive gland and gill tissues of Ruditapes decussatus specimens subjected to varying concentrations of boric acid over a 96-h period under controlled laboratory conditions.
Materıal and Methods
Experimental Design
Samples of R. decussatus were procured from Gelibolu Seafood Import Export Industry, Turkey, a local farm. Upon acquisition, the specimens were acclimated in laboratory conditions within aquariums containing 15 L of artificial seawater for a duration of 5 days, equating to approximately 1 L per mussel. Throughout this acclimation period, the artificial seawater was renewed daily, with a complete replacement on the initial two days, followed by a 50% renewal on the subsequent 3rd and 4th days. The experimental design encompassed three replicates, each comprising 10 individuals per concentration level. The acute effects assessment was conducted over a span of 96 h. The concentrations of exposure (0, 0.05, 0.5, and 5 mg/L) were determined based on established doses from prior literature [3, 34]. All experimental groups were provided with aerated environments ensuring requisite water quality parameters. Monitoring of water temperature and dissolved oxygen levels was performed utilizing a YSI MPS 556 probe, while pH values were routinely assessed employing a HANNA C 200 (HI 83200) photometer. Ethical guidelines were rigorously adhered to throughout the experimental procedures.
Sampling
At the culmination of the exposure duration, ten mussel specimens from each aquarium underwent dissection subsequent to morphological measurements encompassing length, width, and height. Among these samples, five from each experimental group were allocated for histopathological evaluation, while the remaining five were designated for the assessment of antioxidant parameters. Histopathological analyses entailed the examination of mantle, gill, and digestive gland tissues of the mussel specimens. Concurrently, oxidative parameters were ascertained in both gill and digestive gland tissues.
Oxidative Stress Parameters
Gill and digestive gland tissues were promptly fixed with liquid nitrogen upon collection and subsequently stored at − 80 °C until the commencement of analyses. Prior to analysis, tissue homogenization was performed utilizing a 50 mM phosphate buffer. Oxidative parameters, notably the enzymatic activities of superoxide dismutase (SOD), catalase (CAT), and glutathione-S-transferase (GST), alongside the quantification of lipid peroxidation (MDA), were assessed. To standardize enzyme activities in terms of U.mg.protein−1, the protein content within the tissues was quantified employing the Bradford method [48].
SOD activity was evaluated through the reduction of nitroblue tetrazolium (NBT), resulting in the formation of a blue-hued formazan product with maximal absorbance at 550 nm [49, 50]. CAT activity was determined by monitoring alterations in absorbance over a duration of approximately 90 s subsequent to initial tissue measurements [51]. GST activity analysis involved spectrophotometric measurements at 340 nm, taken at distinct time intervals, followed by kinetic computations [52]. Lipid peroxidation, serving as an indicative marker of oxidative damage, was quantified based on the levels of MDA, the terminal product of this oxidative process [53].
Histopathological Assessment
The mantle, gill, and digestive gland tissues of the mussels underwent fixation in Davidson’s fixative for a duration of 24 h, followed by immersion in a 70% ethanol solution. Subsequent to standard histological preparation protocols, tissue embedding in paraffin blocks facilitated the generation of sections measuring 5 µm in thickness. These sections were then subjected to staining with hematoxylin and eosin, as outlined by Gamble and Wilson [54]. Histopathological alterations were meticulously examined, and visual documentation was facilitated through employment of a CX31 Olympus light microscope, equipped with a digital camera, utilizing DP2-BSW software.
Data Analysis
The statistical analyses were conducted utilizing SPSS 21.0 software. The normal distribution of the data was assessed employing the Kolmogorov–Smirnov test, while the homogeneity of variances was evaluated using the Levene test. Enzyme analyses and MDA levels underwent comparison via parametric one-way ANOVA and/or non-parametric Kruskal–Wallis tests. Distinct letters or numbers were assigned to denote significant differences among concentrations. The relationship between quantified histological parameters and oxidative measurements was explored through discriminant analysis, ensuring validation for non-linearity and variances. A significance level (α) of 0.05 was adopted for all analyses.
Results
The morphometric attributes, encompassing length, width, height, and weight measurements of all mussel specimens, are delineated in Table 1. In the study’s inception, a deliberate effort was made to select mussel samples exhibiting comparable lengths and weights, thereby mitigating potential variations stemming from morphological disparities during subsequent analyses.
Oxidative Stress Parameters
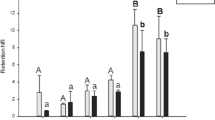
In the control group, the SOD activity in gill tissue samples, devoid of exposure to any concentration of boric acid, exhibited a range between 20.5 and 26.2 U mg.prot.−1, while in the digestive gland tissues, the activity ranged from 4.6 to 15.5 U mg.prot.−1. Conversely, in groups exposed to the lowest concentration of 0.05 mg/L boric acid, the SOD values measured in gill tissues ranged from 31.5 to 49.3 U mg.prot.−1, whereas in digestive gland tissues, this spanned between 17.5 and 60 U mg.prot.−1. Notably, the most elevated SOD enzyme activity values were discerned in digestive gland tissues of specimens subjected to 0.5 mg/L boric acid (168.5 mg.prot.−1). Similarly, heightened values were also observed in gill tissues compared to other concentrations. The observed disparity in SOD levels among concentrations exhibited statistical significance (F = 63.9, df = 3, p < 0.05). Moreover, statistically significant differences were noted in SOD activity among the targeted tissues (F = 4.9, df = 1, p < 0.05) (refer to Fig. 1a).
a SOD, b CAT, c GST activities, and d MDA level in the gill and digestive gland tissues (D.G: digestive gland) of R. decussatus against in vitro boric acid exposures (0, 0.05, 0.5, and 5 mg/L) for 96 h (*the mean difference is significant against the concentrations; **the mean difference is significant against both the concentrations and the tissues; p < 0.05)
The CAT levels in the gill and digestive gland tissues of the control group samples were determined to range between 70 and 157 µmol mg prot.−1. Notably, the highest CAT value was observed in the digestive gland tissue of a specimen exposed to 0.5 mg/L boric acid (770 µmol mg prot−1), with the mean CAT levels being notably elevated across different tissues within this group (mean 488.9 µmol mg prot.−1). Statistical analysis revealed significant differences in CAT levels among concentrations (F = 75.1, df = 3, p < 0.05) (see Fig. 1b).
Conversely, the GST enzyme levels, representing a phase II detoxification enzyme, were initially quantified at 0.06 µmol mg prot.−1 in the gill tissue of the lowest control group. Subsequently, the gill tissue of individuals exposed to 0.5 mg/L boric acid exhibited the highest GST levels (mean 0.12 µmol mg prot.−1), followed by those in tissues of specimens exposed to the highest concentration (5 mg/L) (mean 0.09 µmol mg prot. −1), and then the lowest concentration (mean 0.07 µmol mg prot.−1), respectively. While fluctuations in GST levels did not attain statistical significance at the tissue level (F = 2.05, df = 1, p > 0.05), significant differences were discerned across concentrations (F = 147.1, df = 3, p < 0.05) (refer to Fig. 1c).
Assessed as an index of oxidative damage, lipid peroxidation was manifested through the detection of malondialdehyde (MDA) levels, the end product of this oxidative process. Comparative analysis against the control group revealed elevated MDA levels in both tissues of the exposure groups. Notably, the highest MDA levels were observed in the digestive gland tissue following exposure to 0.5 mg/L boric acid (mean 0.43 µmol mg prot.−1), succeeded by levels in the digestive gland tissue under 5 mg/L exposure (mean 0.4 µmol mg prot.−1). Statistical examination unveiled significant differences in MDA levels across concentrations (F = 10.2, df = 3, p < 0.05) and among tissues (F = 11.1, df = 1, p < 0.05) (refer to Fig. 1d).
Histopathological Assessment
Mantle
No histopathological aberrations were evident in the mantle sections of the control group (refer to Fig. 2a). Nonetheless, hemositic infiltrations were discerned in the mantle sections of the cohort exposed to 0.05 mg/L boric acid (refer to Fig. 2b). Subsequent observations indicated the pervasiveness of this finding across other doses (0.5 and 5 mg/L) throughout the study (refer to Fig. 2c, d).
Gill
The gill sections of mussels in the control group exhibited a histologically normal appearance (refer to Fig. 3a). Notably, no histopathological anomalies were noted across any of the administered doses (refer to Fig. 3b–d).
Digestive Gland
The digestive gland sections of mussels within the control group displayed histologically normal tubular structures (refer to Fig. 4a). However, specimens exposed to 0.05 mg/L of boric acid exhibited localized hemositic infiltrations (refer to Fig. 4b). Notably, the pervasiveness of hemositic infiltrations increased notably in the group subjected to 0.5 mg/L boric acid exposure (refer to Fig. 4c). Furthermore, specimens exposed to 5 mg/L of boric acid showcased pronounced hemositic infiltrations along with epithelial deformations within the digestive gland tubules (refer to Fig. 4d).
Discussion
Boric acid, one of the twelve naturally occurring boron-containing compounds [55], is widely employed for its therapeutic attributes in addressing inflammatory conditions [56]. Its historical use as a pesticide in agricultural practices spans many years [21, 57], and reports also indicate its antifungal or fungistatic properties [58,59,60]. Given its application as an inorganic chemical insecticide, studies have revealed that boric acid can disrupt specific physiological and biochemical processes in non-target organisms [5, 61, 62].
In investigations spanning both vertebrate and invertebrate taxa, boric acid has been observed to manifest among the lowest degrees of bioaccumulation and associated potential toxicities [59]. Studies concerning boron toxicity predominantly emphasize developmental biology [24]. Various inquiries targeting diverse fly species [63], assorted insect taxa [64, 65], and even human subjects [66] have delineated adverse outcomes linked to boron and its derivatives across distinct developmental stages. Illustrating aquatic ecosystems, observations have indicated variances in the growth of certain fish species correlating with boron concentrations [37, 38].
When considering the potential impact of substances introduced into aquatic ecosystems, it becomes evident that they may manifest toxic effects over time owing to bioaccumulation, thereby disrupting the ecosystem’s functionality and adversely affecting organisms across various trophic levels [67]. It is noteworthy that the manifestation of toxicity in aquatic organisms can exhibit variability contingent upon the specific species involved [12, 68]. Concurrently, research endeavors assessing the toxicity of boron and its derivatives in aquatic organisms, in conjunction with growth factors, have been documented. In the realm of acute exposures, lethal concentrations (LC50) have been delineated for boron and its derivatives across diverse fish species. These concentrations were elucidated as 74 mg/L for dab (Limanda limanda) [69], 43 mg/L for coho salmon (Oncorhynchus kisutch) [70], 979 mg/L for mosquito fish (Gambusia affinis) [71], 108–252 mg/L for flounder (Paralichthys olivaceus), and 97–172 mg/L for sea bream (Parus major) [18].
In a prior investigation, it was observed that boron derivative concentrations below 10 mg/L did not manifest toxic effects on trout species [2]. Leveraging this observation, the current study aimed to evaluate the histological ramifications and quantifiable physiological responses induced by borax acid, a widely utilized substance spanning diverse domains, on specimens of R. decussatus, a bivalve species integral to human consumption. Employing a meticulously devised experimental framework, artificial seawater was meticulously concocted, facilitating a 96-h exposure of the samples to borax acid. Histological assessments unveiled an absence of cellular alterations in specimens exposed to concentrations below 5 mg/L, contrasting starkly with pronounced signs of hemositic infiltration and epithelial deformation observed at the highest concentration. The escalating prevalence of histological irregularities in tandem with concentration corroborates prior research illustrating the toxicity profile of boron and its derivatives [22, 72,73,74].
Numerous studies have been conducted to evaluate the extent of oxidative damage and genotoxicity induced by boron and its derivatives. Particularly within investigations involving mammalian groups, it has been documented that boron and its derivatives elicit increases in antioxidant levels, while the ensuing damage lacks genotoxicity [41, 75,76,77,78,79,80,81]. Nonetheless, despite the myriad of evaluations undertaken, definitive establishment of the effect of boron and its derivatives on antioxidants remains inconclusive [4]. In an effort to elucidate the antioxidant defense system concerning potential physiological or pathological conditions that may ensue in mussel samples subsequent to exposure, levels of SOD, CAT, and GST enzymes were scrutinized, alongside the assessment of LPO quantity to gauge oxidative damage. It is envisaged that concomitant with the escalation of reactive oxygen species (ROS) upon exposure, the delicate balance of antioxidants will be disrupted [82]. Furthermore, the stress response that mussels may exhibit to varying concentrations of boric acid is favored due to its propensity to perturb normal body homeostasis, culminating in an array of biochemical, physiological, and behavioral alterations.
At concentrations ranging up to a maximum of 5 mg/L, evidence suggests the potential initiation of oxidative damage in two distinct tissue types within the samples. Following acute exposure to boric acid, notably heightened antioxidant levels were discerned at a concentration of 0.5 mg/L, denoting a moderate concentration level. Noteworthy is the observation that the pinnacle levels of enzymes catalyzing Phase I reactions, such as SOD and CAT, were predominantly present in the digestive gland tissue. Conversely, the GST enzyme, instrumental in Phase II reactions, exhibited its highest values within the gill tissue. This observed variance between tissue types may be correlated with the gill tissue’s precedent exposure to boric acid, which appears to be both earlier and more extensive.
The prompt response exhibited by the SOD enzyme within this context can be attributed to its capacity to uphold the primary line of defense without necessitating an increase in the prevailing metabolic energy reservoirs of mussels [83]. Analogously rapid SOD responses have been documented in exposure investigations encompassing diverse mussel species subsequent to pollutant exposure [84,85,86]. Moreover, varying concentrations of boron compounds have been shown to induce heightened SOD levels [75]. Nevertheless, contrary to initial expectations, this study revealed that SOD levels did not exhibit a linear increment with escalating concentrations of boric acid; rather, a tendency towards reduction was noted at higher concentrations, aligning with findings in extant literature [4, 77]. It could be posited that this declining trend might be associated with the depletion of detoxification mechanisms [87, 88].
The concentrations of CAT and GST displayed a progressive increase from the lowest to the moderate levels, mirroring the trend observed in SOD activity, yet exhibited a decline to lower levels at the highest concentration. The elevation in CAT activity can be attributed to its defensive role against oxygen radicals generated during exposure to boric acid [4]. Conversely, the heightened catalase (CAT) activity in response to increased hydrogen peroxide (H2O2) levels in both tissue types of boric acid-exposed specimens implies the presence of exposure-induced redox imbalance [89]. Considering the cooperative action of CAT and GST enzymes against oxidative stress in both the gill and digestive gland tissues, the concentration-based results remain consistent. However, notably, disparate increases in enzyme activities across different organs were particularly evident at the 0.5 mg/L exposure level. This finding at the 0.5 mg/L boric acid exposure strengthens the notion that the initial response in gill tissue entails GST activity, whereas in the digestive gland tissue, it involves CAT induction under the same exposure conditions [90]. These increments in enzyme levels substantiate the occurrence of oxidative stress consequent to boric acid exposure in mussels, stemming from an imbalance in pro-/antioxidant metabolism, thus supporting the concordance of oxidative effects associated with boric acid toxicity with prior research [4, 34, 41, 75,76,77,78,79,80,81].
When faced with a contaminant, the structural integrity of cell membranes can be compromised, leading to the deactivation of membrane-associated enzymes and receptors, a phenomenon termed lipid peroxidation [91]. Levels of lipid peroxidation serve as specific indicators of the activity status of antioxidant systems [92,93,94]. In the current investigation, the probability of encountering a moderate oxidative stress scenario resulting from the elevation in tissue MDA levels attributed to boric acid exposure has been reinforced. Measurements taken in the digestive gland tissues exhibited significantly higher values across all three concentrations. The highest LPO values were documented at a concentration of 0.5 mg/L. Prior studies have also documented escalated lipid peroxidation phenomena in aquatic organisms following exposure to pollutants [95,96,97,98,99,100,101,102,103].
This study aimed to assess the toxic potential of boric acid, which is extensively utilized across various sectors, in R. decussatus, an integral species in the food chain due to its filter-feeding behavior. The investigation focused on elucidating the potential adverse effects of boric acid by examining histopathological changes and antioxidant responses upon its introduction into aquatic ecosystems. The findings revealed notable physiological and specific histological alterations in mussels as a result of boric acid exposure. Considering its industrial and agricultural utility, the study underscores the importance of judicious boric acid usage to mitigate potential harms and ecological ramifications.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Moseman RF (1994) Chemical disposition of boron in animals and humans. In: Environmental Health Perspectives. vol 102. https://doi.org/10.1289/ehp.94102s7113
Eckhert CD (1998) Boron stimulates embryonic trout growth. J Nutr 128(12). https://doi.org/10.1093/jn/128.12.2488
Li E, Xiong Z, Chen L, Zeng C, Li K (2008) Acute toxicity of boron to juvenile white shrimp, Litopenaeus vannamei, at two salinities. Aquaculture 278(1–4). https://doi.org/10.1016/j.aquaculture.2008.03.022
Alak G, Ucar A, Parlak V, et al (2021) Antioxidant potential of ulexite in zebrafish brain: assessment of oxidative DNA damage, apoptosis, and response of antioxidant defense system. Biol Trace Elem Res 199(3). https://doi.org/10.1007/s12011-020-02231-7
Radwan MA, Gad AF (2023) Exploring the mechanisms underlying the toxicity of boric acid against the land snail, Theba pisana. Pest Manage Sci 79(5). https://doi.org/10.1002/ps.7342
Warington K (1923) The effect of boric acid and borax on the broad bean and certain other plants. Ann Botany os-37(4). https://doi.org/10.1093/oxfordjournals.aob.a089871
Öz M, Yavuz O, Bolukbas F (2020) Histopathology changes in the rainbow trout (Onchorhyncus mykiss) consuming boric acid supplemented fish fodder. J Trace Elem Med Biol 62. https://doi.org/10.1016/j.jtemb.2020.126581
Hunter JM, Nemzer B v., Rangavajla N, et al (2019) The fructoborates: part of a family of naturally occurring sugar–borate complexes—biochemistry, physiology, and ımpact on human health: a review. Biol Trace Elem Res 188(1). https://doi.org/10.1007/s12011-018-1550-4
Devirian TA, Volpe SL (2003) The physiological effects of dietary boron. Crit Rev Food Sci Nutr 43(2). https://doi.org/10.1080/10408690390826491
(2013) Scientific Opinion on the re-evaluation of boric acid (E 284) and sodium tetraborate (borax) (E 285) as food additives. EFSA J. 11(10). https://doi.org/10.2903/j.efsa.2013.3407
Yildiz G, Koksal BH, Sizmaz O (2013) Effects of dietary boric acid addition on growth performance, cholesterolemia, some carcass and tibia characteristics in different rearing periods in broiler chickens. Revue de Medecine Veterinaire 164(4):219–224
Gülsoy N, Yavaş C, Mutlu Ö (2015) Genotoxic effects of boric acid and borax in zebrafish, danio rerio using alkaline comet assay. EXCLI J 14. https://doi.org/10.17179/excli2015-404
Nielsen FH, Meacham SL (2011) Growing evidence for human health benefits of boron. J Evidence-Based Complement Alternat Med 16(3). https://doi.org/10.1177/2156587211407638
Nielsen FH (2014) Update on human health effects of boron. J Trace Elem Med Biol 28(4). https://doi.org/10.1016/j.jtemb.2014.06.023
Helvacı C (2003) Türkiye borat yatakları jeolojik konumu, ekonomik önemi ve bor politikası. J Balikesir Univ Inst Sci Technol 5(1):4–41
Zumreoglu-Karan B, Kose DA (2015) Boric acid: a simple molecule of physiologic, therapeutic and prebiotic significance. In: Pure and Applied Chemistry. vol 87. https://doi.org/10.1515/pac-2014-0909
Hamilton SJ, Buhl KJ (1990) Acute toxicity of boron, molybdenum, and selenium to fry of chinook salmon and coho salmon. Arch Environ Contam Toxicol 19(3). https://doi.org/10.1007/BF01054980
Furuta T, Iwata N, Kikuchi K (2007) Effects of fish size and water temperature on the acute toxicity of boron to Japanese flounder Paralichthys olivaceus and red sea bream Pagrus major. Fish Sci 73(2). https://doi.org/10.1111/j.1444-2906.2007.01342.x
Akar D (2007) Potential boron pollution in surface water, crop, and soil in the lower Buyuk Menderes Basin. Environ Eng Sci 24(9). https://doi.org/10.1089/ees.2006.0218
Xu RJ, Xing XR, Zhou QF, Jiang G bin, Wei FS (2010) Investigations on boron levels in drinking water sources in China. Environ Monit Assess 165(1–4). https://doi.org/10.1007/s10661-009-0923-8
See AS, Salleh AB, Bakar FA, Yusof NA, Abdulamir AS, Heng LY (2010) Risk and health effect of boric acid. Am J Appl Sci 7(5). https://doi.org/10.3844/ajassp.2010.620.627
Ismail HTH (2022) Toxic effects of excess exposure to boric acid on serum biochemical aspect, hematology and histological alterations and ameliorative potential role of melatonin in rats. Saudi J Biol Sci 29(10). https://doi.org/10.1016/j.sjbs.2022.103425
Schou JS, Jansen JA, Aggerbeck B (1984) Human pharmacokinetics and safety of boric acid. Arch Toxicol 55(SUPPL. 7). https://doi.org/10.1007/978-3-642-69132-4_32
Hadrup N, Frederiksen M, Sharma AK (2021) Toxicity of boric acid, borax and other boron containing compounds: a review. Regul Toxicol Pharmacol 121. https://doi.org/10.1016/j.yrtph.2021.104873
Krasovskii GN, Varshavskaya SP, Borisov AI (1976) Toxic and gonadotropic effects of cadmium and boron relative to standards for these substances in drinking water. Environ Health Perspect 13. https://doi.org/10.1289/ehp.761369
Treinen KA, Chapin RE (1991) Development of testicular lesions in F344 rats after treatment with boric acid. Toxicol Appl Pharmacol 107(2). https://doi.org/10.1016/0041-008X(91)90212-W
Ku WW, Chapin RE, Wine RN, Gladen BC (1993) Testicular toxicity of boric acid (BA): relationship of dose to lesion development and recovery in the F344 rat. Reprod Toxicol 7(4). https://doi.org/10.1016/0890-6238(93)90020-8
Xing X, Wu G, Wei F et al (2008) Biomarkers of environmental and workplace boron exposure. J Occup Environ Hyg 5(3). https://doi.org/10.1080/15459620701845132
Schoderboeck L, Mühlegger S, Losert A, Gausterer C, Hornek R (2011) Effects assessment: boron compounds in the aquatic environment. Chemosphere 82(3). https://doi.org/10.1016/j.chemosphere.2010.10.031
Ball RW, Harrass MC, Culver BD (2012) Boron. Patty’s Toxicol 45:885–934
Genç TO, İnanan BE, Yabanlı M, Yılmaz F (2015) Japon Balığı (Carassius auratus Linnaeus, 1758) Dokularında Bor Akümülasyonu. Turkish J Agric - Food Sci Technol 3(6). https://doi.org/10.24925/turjaf.v3i6.498-503.296
Acar Ü, İnanan BE, Zemheri F, Kesbiç OS, Yılmaz S (2018) Acute exposure to boron in Nile tilapia (Oreochromis niloticus): median-lethal concentration (LC50), blood parameters, DNA fragmentation of blood and sperm cells. Chemosphere 213. https://doi.org/10.1016/j.chemosphere.2018.09.063
Alak G, Ucar A, Yeltekin AÇ et al (2018) Neuroprotective effects of dietary borax in the brain tissue of rainbow trout (Oncorhynchus mykiss) exposed to copper-induced toxicity. Fish Physiol Biochem 44(5). https://doi.org/10.1007/s10695-018-0530-0
Alak G, Parlak V, Ucar A et al (2020) Oxidative and DNA damage potential of colemanite on xebrafish: brain, liver and blood. Turkish J Fish Aquatic Sci 20(8). https://doi.org/10.4194/1303-2712-v20_8_02
Öz M, Dikel S, İnanan BE, Karaşahin T, Durmuş M, Uçar Y (2017) The effects of boric acid on the hepatosomatic and viserosomatic index values of rainbow trout. J Adv VetBio Sci Tech 2(1):6–10
Öz M, Inanan BE, Dikel S (2018) Effect of boric acid in rainbow trout (Oncorhynchus mykiss) growth performance. J Appl Anim Res 46(1). https://doi.org/10.1080/09712119.2018.1450258
Öz M, Tatil T, Dikel S (2021) Effects of boric acid on the growth performance and nutritional content of rainbow trout (Oncorhynchus mykiss). Chemosphere 272:129895
İnanan BE, Yılmaz F (2018) Motility evaluation and cryopreservation of fish sperm exposed by water-borne and food-borne boron. J Aquac Eng Fish Res. https://doi.org/10.3153/jaefr18002
Comba B, Oto G, Mis L, Özdemir H, Comba A (2016) Effects of borax on inflammation, haematological parameters and total oxidant-antioxidant status in rats applied 3–methylcholanthrene. Kafkas Universitesi Veteriner Fakultesi Dergisi. https://doi.org/10.9775/kvfd.2016.15001
Acaroz U, Ince S, Arslan-Acaroz D, et al (2019) Bisphenol-A induced oxidative stress, inflammatory gene expression, and metabolic and histopathological changes in male Wistar albino rats: Protective role of boron. Toxicol Res 8(2). https://doi.org/10.1039/c8tx00312b
Pawa S, Ali S (2006) Boron ameliorates fulminant hepatic failure by counteracting the changes associated with the oxidative stress. Chemico-Biol Interact 160(2). https://doi.org/10.1016/j.cbi.2005.12.002
Keren R, Bingham FT (1958) Boron in water, soils, and plants. In. https://doi.org/10.1007/978-1-4612-5046-3_7
Regoli F, Gorbi S, Frenzilli G et al (2002) Oxidative stress in ecotoxicology: from the analysis of individual antioxidants to a more integrated approach. Mar Environ Res 54(3–5). https://doi.org/10.1016/S0141-1136(02)00146-0
Regoli F, Pellegrini D, Winston GW et al (2002) Application of biomarkers for assessing the biological impact of dredged materials in the Mediterranean: the relationship between antioxidant responses and susceptibility to oxidative stress in the red mullet (Mullus barbatus). Mar Pollut Bullet 44(9). https://doi.org/10.1016/S0025-326X(02)00120-0
Vlahogianni T, Dassenakis M, Scoullos MJ, Valavanidis A (2007) Integrated use of biomarkers (superoxide dismutase, catalase and lipid peroxidation) in mussels Mytilus galloprovincialis for assessing heavy metals’ pollution in coastal areas from the Saronikos Gulf of Greece. Mar Pollut Bullet 54(9). https://doi.org/10.1016/j.marpolbul.2007.05.018
Bebianno MJ, Géret F, Hoarau P et al (2004) Biomarkers in Ruditapes decussatus: a potential bioindicator species. Biomarkers 9(4–5). https://doi.org/10.1080/13547500400017820
Cravo A, Lopes B, Serafim A et al (2013) Spatial and seasonal biomarker responses in the clam Ruditapes decussatus. Biomarkers 18(1). https://doi.org/10.3109/1354750X.2012.730549
Bradford MM (1976) Determinación de proteínas: método de bradford. Anal Biochem 254:248–254
Flohé L, ötting F (1984) [10] Superoxide dismutase assays. Methods in enzymology 105(C). https://doi.org/10.1016/S0076-6879(84)05013-8
Cigremiş Y (1997) Sigara Tiryakilerinde Eritrosit İçi Süperoksit Dismutaz, Katalaz ve Glutatyon Peroksidaz Aktivite Düzeyleri. Master Dissertation (Yüksek Lisans Tezi). İnönü Üniversitesi, Türkiye
Clairborne A (1985) Catalase activity. In: Grenwald RA (ed) Handbook of methods of oxygen radical research. CRC Press, Boca Raton, FL, pp 283–284
Gibson GG, Skett P (1996) Factors affecting drug metabolism: internal factors. In: Introduction to drug metabolism. https://doi.org/10.1007/978-1-4899-6844-9_4
Jain SK, McVie R, Duett J, Herbst JJ (1989) Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes 38(12). https://doi.org/10.2337/diab.38.12.1539
Gamble M, Wilson I (2008) The hematoxylins and eosin. Theory Pract Histological Tech 6:121–134
Farfán-García ED, Castillo-García EL, Soriano-Ursúa MA (2018) More than boric acid: increasing relevance of boron in medicine. World J Transl Med 7(1). https://doi.org/10.5528/wjtm.v7.i1.1
Turk T, Kaval ME, Şen BH (2015) Evaluation of the smear layer removal and erosive capacity of EDTA, boric acid, citric acid and desy clean solutions: an in vitro study. BMC Oral Health 15(1). https://doi.org/10.1186/s12903-015-0090-y
Sabbour MM, Abdel-Hakim EA, Abdou WL (2012) Role of some additives in enhancing the formulation of bacteria Bacillus thuringiensis against Phthorimaea operculella and Helicoverpa armigera: 2-chemical additives. J Appl Sci Res 2640–2649
de Seta F, Schmidt M, Vu B, Essmann M, Larsen B (2009) Antifungal mechanisms supporting boric acid therapy of Candida vaginitis. J Antimicrob Chemother 63(2). https://doi.org/10.1093/jac/dkn486
J.S G, S.S G, N.A K (2021) Boric acid toxicity in some selected molluscan species. Int J Biol Environ Investig 1(1). https://doi.org/10.33745/ijbei.2021.v01i01.005
Khairy M, Ayoub HA, Rashwan FA, Abdel-Hafez HF (2021) Sea urchin-like calcium borate microspheres and synergistic action with cholinesterase-inhibiting insecticides for ecofriendly: Spodoptera littoralis control. Environ Sci: Processes Impacts 23(7). https://doi.org/10.1039/d1em00125f
Białek M, Czauderna M, Krajewska KA, Przybylski W (2019) Selected physiological effects of boron compounds for animals and humans. A review. J Anim Feed Sci 28(4). https://doi.org/10.22358/jafs/114546/2019
Büyükgüzel E, Büyükgüzel K, Snela M et al (2013) Effect of boric acid on antioxidant enzyme activity, lipid peroxidation, and ultrastructure of midgut and fat body of Galleria mellonella. Cell Biol Toxicol 29(2). https://doi.org/10.1007/s10565-013-9240-7
AA Amin, MH Soliman, AA Shalaby (2017) Evaluation of the efficiency of certain attractive toxic baits for the control of the house fly, Musca domestica (Diptera: Muscidac). Chem Sci J 08(04). https://doi.org/10.4172/2150-3494.1000170
Khan KA (2006) Enhancement of virulence of Bacillus thuringiensis and Serratia marcescens by chemicals. J Res (Sci) 17(1):35–44
Habes D, Messiad R, Gouasmia S, Grib L (2013) Effects of an inorganic insecticide (boric acid ) against Blattella germanica: morphometric measurements and biochemical composition of ovaries. African J Biotechnol 12(18):2492–2497. https://doi.org/10.5897/AJB2012.2953
Faza AS, Dewanti L, Ema Q (2020) Effects of low dose exposure of Borax for 8 weeks on gastric ulcer formation. Majalah Biomorfologi 30(1):7–13. https://doi.org/10.20473/mbiom.v30i1.2020.7-13
Londhe S, Patil S, Kamble N (2015) Effect of mercuric chloride on terrestrial slug Semperula maculata and histopathology of reproductive organs: a comprehensive study. Toxicol Environ Chem 97(2). https://doi.org/10.1080/02772248.2015.1046680
Birge WJ, Black JA (1977) Sensitivity of vertebrate embryos to boron compounds. EPA-560/1–76–008. Office of Toxic Substances, USEPA, Washington, D.C
Taylor D, Maddock BG, Mance G (1985) The acute toxicity of nine “grey list” metals (arsenic, boron, chromium, copper, lead, nickel, tin, vanadium and zinc) to two marine fish species: dab (Limanda limanda) and grey mullet (Chelon labrosus). Aquatic Toxicol 7(3). https://doi.org/10.1016/S0166-445X(85)80001-1
Thompson JAJ, Davis JC, Drew RE (1976) Toxicity, uptake and survey studies of boron in the marine environment. Water Res 10(10). https://doi.org/10.1016/0043-1354(76)90021-X
Wallen IE, Greer WC, Lasater R (1957) Pollution to “Gambusia affinis” of certain pure chemicals in turbid waters. Sewage Indust Wastes 29(6):695–711
Sabuncuoglu BT, Kocaturk PA, Yaman O, Kavas GO, Tekelioglu M (2006) Effects of subacute boric acid administration on rat kidney tissue. Clin Toxicol (Phila) 44(3):249–253. https://doi.org/10.1080/15563650600584386
Ayranci DFE, Ozelmas U, Ayranci U (2021) Histopathological changes on testes, liver, kidney and brain tissues in acute boric acid administration. J Pharm Res Int 33(47B):337–346. https://doi.org/10.9734/jpri/2021/v33i47B33133
Abdel Aliem R et al (2022) Toxicological effect of boric acid and cadmium chloride on the Nile tilapia, Oreochromis niloticus. Egyptian J Aquatic Biol Fish 26(5):667–680
Nielsen FH (1994) Biochemical and physiologic consequences of boron deprivation in humans. In: Environmental health perspectives. vol 102. https://doi.org/10.1289/ehp.94102s759
Hunt CD (1998) Regulation of enzymatic activity: one possible role of dietary boron in higher animals and humans. In: Biological Trace Element Research. vol 66. https://doi.org/10.1007/BF02783139
Türkez H, Geyikoǧlu F, Tatar A, Keleş S, Özkan A (2007) Effects of some boron compounds on peripheral human blood. Zeitschrift fur Naturforschung - Section C J Biosci 62(11–12). https://doi.org/10.1515/znc-2007-11-1218
Geyikoglu F, Turkez H (2008) Boron compounds reduce vanadium tetraoxide genotoxicity in human lymphocytes. Environ Toxicol Pharmacol 26(3). https://doi.org/10.1016/j.etap.2008.07.002
Alak G, Atamanalp M, Topal A, Arslan H, Oruç E, Altun S (2013) Histopathological and biochemical effects of humic acid against cadmium toxicity in brown trout gills and muscles. Turkish J Fish Aquatic Sci 13(2). https://doi.org/10.4194/1303-2712-v13_2_13
Ince S, Kucukkurt I, Demirel HH, Acaroz DA, Akbel E, Cigerci IH (2014) Protective effects of boron on cyclophosphamide induced lipid peroxidation and genotoxicity in rats. Chemosphere 108. https://doi.org/10.1016/j.chemosphere.2014.01.038
Üstündaǧ A, Behm C, Föllmann W, Duydu Y, Degen GH (2014) Protective effect of boric acid on lead- and cadmium-induced genotoxicity in V79 cells. Arch Toxicol 88(6). https://doi.org/10.1007/s00204-014-1235-5
Brucka-Jastrzębska E (2010) The effect of aquatic cadmium and lead pollution on lipid peroxidation and superoxide dismutase activity in freshwater fish. Pol J Environ Stud 19(6):1139–1150
Andrade M, Soares AM, Solé M, Pereira E, Freitas R (2023) Threats of pollutants derived from electronic waste to marine bivalves: the case of the rare-earth element yttrium. Environ Toxicol Chem 42(1):166–177
Pinto J, Costa M, Leite C, Borges C, Coppola F, Henriques B, Monteiro R, Russo T, Di Cosmo A, Soares AMVM, Polese G, Pereira E, Freitas R (2019) Ecotoxicological effects of lanthanum in Mytilus galloprovincialis: biochemical and histopathological impacts. Aquat Toxicol 211:181–192. https://doi.org/10.1016/j.aquatox.2019.03.017
Freitas R, Cardoso CED, Costa S, Morais T, Moleiro P, Lima AFD, Soares M, Figueiredo S, Águeda TL, Rocha P, Amador G, Soares AMVM, Pereira E (2020a) New insights on the impacts of e-waste towards marine bivalves: the case of the rare earth element dysprosium. Environ Pollut 260, Article 113859. https://doi.org/10.1016/j.envpol.2019.113859
Freitas R, Costa S, Cardoso CED, Morais T, Moleiro P, Matias AC, Pereira AF, Machado J, Correia B, Pinheiro D, Rodrigues A, Colónia J, Soares AMVM, Pereira E (2020b) Toxicological effects of the rare earth element neodymium in Mytilus galloprovincialis. Chemosphere, 244, Article 125457. https://doi.org/10.1016/j.chemosphere.2019.125457
Jacobson SV, Reimschuessel R (1998) Modulation of superoxide production in goldfish (Carassius auratus) exposed to and recovering from sublethal copper levels. Fish Shellfish Immunol 8:245–259
Ertürk Gürkan S, Gürkan M (2021) Toxicity of gamma aluminium oxide nanoparticles in the Mediterranean mussel (Mytilus galloprovincialis): histopathological alterations and antioxidant responses in the gill and digestive gland. Biomarkers 26(3):248–259
da Costa Araújo AP, da Luz TM, Rocha TL, Ahmed MAI, e Silva DDM, Rahman MM, Malafaia G (2022) Toxicity evaluation of the combination of emerging pollutants with polyethylene microplastics in zebrafish: perspective study of genotoxicity, mutagenicity, and redox unbalance. J Hazard Mater 432:128691
Parra S, Varandas S, Santos D, Félix L, Fernandes L, Cabecinha E, ... Monteiro SM (2021) Multi-biomarker responses of asian clam Corbicula fluminea (Bivalvia, Corbiculidea) to cadmium and microplastics pollutants. Water 13(4):394
Kang DH (2002) Oxidative stress, DNA damage, and breast cancer. AACN Clin Issues 13(4). https://doi.org/10.1097/00044067-200211000-00007
Oropesa AL, García-Cambero JP, Soler F (2009) Glutathione and malondialdehyde levels in common carp after exposure to simazine. Environ Toxicol Pharmacol 27(1). https://doi.org/10.1016/j.etap.2008.08.003
Li XY, Luo YR, Yun MX, Wang J, Wang JJ (2010) Effects of 1-methyl-3-octylimidazolium bromide on the anti-oxidant system of earthworm. Chemosphere 78(7). https://doi.org/10.1016/j.chemosphere.2009.11.047
Chen X, Wang X, Gu X, Jiang Y, Ji R (2017) Oxidative stress responses and insights into the sensitivity of the earthworms Metaphire guillelmi and Eisenia fetida to soil cadmium. Sci Total Environ 574. https://doi.org/10.1016/j.scitotenv.2016.09.059
di Giulio RT, Habig C, Gallagher EP (1993) Effects of Black Rock Harbor sediments on indices of biotransformation, oxidative stress, and DNA integrity in channel catfish. Aquatic Toxicol 26(1–2). https://doi.org/10.1016/0166-445X(93)90002-I
Livingstone DR, Lemaire P, Matthews A, Peters L, Bucke D, Law RJ (1993) Pro-oxidant, antioxidant and 7-ethoxyresorufin O-deethylase (EROD) activity responses in liver of dab (Limanda limanda) exposed to sediment contaminated with hydrocarbons and other chemicals. Mar Pollut Bullet 26(11). https://doi.org/10.1016/0025-326X(93)90498-9
Solé M, Porte C, Biosca X et al (1996) Effects of the “Aegean Sea” oil spill on biotransformation enzymes, oxidative stress and DNA-adducts in digestive gland of the mussel (Mytilus edulus L.). Comp Biochem Physiol - C Pharmacol Toxicol Endocrinol 113(2). https://doi.org/10.1016/0742-8413(95)02095-0
Elia AC, Waller WT, Norton SJ (2002) Biochemical responses of bluegill sunfish (Lepomis macrochirus, Rafinesque) to atrazine induced oxidative stress. Bull Environ Contam Toxicol 68:809–816
Huang L, Lu D, Diao J, Zhou Z (2012) Enantioselective toxic effects and biodegradation of benalaxyl in Scenedesmus obliquus. Chemosphere 87(1). https://doi.org/10.1016/j.chemosphere.2011.11.029
Li Y, Zhang S, Jiang W, Liu D (2013) Cadmium accumulation, activities of antioxidant enzymes, and malondialdehyde (MDA) content in Pistia stratiotes L. Environ Sci Pollut Res 20(2). https://doi.org/10.1007/s11356-012-1054-2
Günal AÇ, Tunca SK, Arslan P, Gül G, Dinçel AS (2021) How does sublethal permethrin effect non-target aquatic organisms? Environ Sci Pollut Res 28(37). https://doi.org/10.1007/s11356-021-14475-4
Jiang Q, Jiang Z, Ao S et al (2021) Multi-biomarker assessment in the giant freshwater prawn Macrobrachium rosenbergii after deltamethrin exposure. Ecotoxicol Environ Saf 214. https://doi.org/10.1016/j.ecoenv.2021.112067
Ran L, Yang Y, Zhou X, Jiang X, Hu D, Lu P (2021) The enantioselective toxicity and oxidative stress of dinotefuran on zebrafish (Danio rerio). Ecotoxicol Environ Saf 226. https://doi.org/10.1016/j.ecoenv.2021.112809
Acknowledgements
The authors would like to thank Gelibolu Seafood Company, from which the study material was provided.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation (Volkan Sarıtunç and Ezgi Can İbiş), data collection (Volkan Sarıtunç and Berkay Güneş), and analysis were performed by Mert Gürkan and Selin Ertürk Gürkan. The first draft of the manuscript was written by Selin Ertürk Gürkan, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This is an observational study. The COMU Research Ethics Committee has confirmed that no ethical approval is required.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ertürk Gürkan, S., Gürkan, M., Sarıtunç, V. et al. Evaluation of Possible Toxic Effects of Boric Acid in Palourde Clam (Ruditapes decussatus) Through Histological Changes and Oxidative Responses. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04230-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04230-4