Abstract
Metal pollution is a major environmental concern worldwide, especially in Egypt. The aquaculture industry uses widespread artificial feeds to stimulate fish production, leading to metal accumulation in the aquatic environment. Heavy metal concentrations (HMCs) in sediments, water, and tissues were studied to study the effect of pollution levels on heamatological, and biochemical, immunological aspects of farmed fish as well as on human health. Results declared that the HMC levels in the water and sediment were significantly different between El-Sharkia and Kafr El-Sheikh fishponds (T-test, p < 0.05). This was supported by the metal pollution index in the water and sediment, indicating that El-Sharkia fishponds (ES fishponds) were more contaminated than Kafr El-Sheikh fishponds (KES fishponds). Also, HMCs in fish tissues were significantly increased in fish cultivated in ES fishponds than in KES fishponds. Haematological, immunological, and biochemical alterations of Bolti (Oreochromis niloticus) and Topara (Chelon ramada) fish were significantly different within the different fish species as well as the different fishponds. From the human health perspective, the THQ-HMC and HI-HMC associated with the consumption of muscle suggest a safe non-carcinogenic risk to human health. In contrast, cadmium poses a cancer risk to children who consume the muscular tissue of Bolti fish from ES fishponds, which should be regarded as a warning sign based on data indices and a human health perspective. In order to minimise HMC pollution in the aquaculture sector, it is advisable to take possible assessments and carry out continuous monitoring considering international WHO/FAO assessments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture production is increasing every year worldwide and in 2018, 179 million tons of aquaculture output were produced. It is believed that increasing fish production is the only possible option to close the gap between protein production and consumption [26, 41]. In Egypt, the majority of aquaculture farms use Bolti (Oreochromis niloticus) and Topara (Chelon ramada) as part of their polyculture practices [28, 30].
The HMCs are present in aquatic habitats in a variety of ways, including through untreated or insufficiently treated agricultural, domestic, and industrial effluent [80]. Metals are ingested by aquatic organisms in small amounts through water uptake and in larger amounts through biomagnification of prey,however, consumers can ingest metals through the food chain, which can have acute and long-term health effects [3]. Egyptian aquaculture farms serve as temporary reservoirs for wastewater Soltan et al. [61], with the aquatic habitat and its water quality being the primary determinants of fish health or disease. One of the main threats to aquatic organisms is pollution of the aquatic environment by inorganic and organic pollutants [57].
The assessment of the quality of the aquatic environment via monitoring the levels of HMC pollution by determining their levels in sediment, water, and tissues of fish [9, 34]. The fish HMCs are significantly influenced by several factors, and identifying fish species with elevated levels of metals is very important for consumer awareness and safety The level of contamination varies by fish species, source of contamination, trophic level, collection site, and type of feeding [74]. Physiological and biochemical indices in aquatic organisms are widely used as biomarkers to assess their health [23, 29]. Due to the risks to human health connected with the bioaccumulation of HMC through fish intake, the monitoring of HMC in fish and fish farm environments has become crucial. Consequently, it is important to determine the HMC in the muscles of cultivated fish to assess the potential risks of their intake for humans. Therefore, this study aims to (i) evaluate the quality status of the polyculture ponds (KES and ES fishponds) regarding ecotoxic HMC considering three interrelated aquatic compartments: water, sediments, and fish species )Botti, and Topara(, (ii) study the effect of pollution levels on heamatological, biochemical, and immunological, aspects of ponds fish and (iii) to evaluate the possible human health hazards associated with ingestion of muscle of ponds species by applying the non-carcinogenic and carcinogenic equations.
Material and Methods
Sampling Fishponds
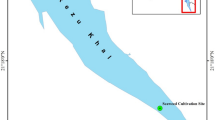
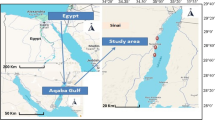
This investigation was performed at the most productive aquaculture sites in Egypt; the first farms were in the ES governorates (n = 7 ES fishponds, total of 18 feddan) at latitude and longitude (30.67305450 and 31.15932470, respectively), and the second ponds were in the KES governorates (n = 8 KES fishponds, total of 20 feddan) at latitude and longitude (31.30854440 and 30.80394740, respectively). Bolti fish is the local name of tilapia species (Oreochromis niloticus) while Topara fish is the local name of mullet species (Chelon ramada). Fish from both ponds were cultivated in polyculture and semi-intensive ponds and received their freshwater from the agricultural drainage system (Fig. 1). Bolti fish samples have an average weight and standard length of 271.26 ± 16.67 g and 18.17 ± 5.67 cm, respectively while, Topara fish samples have average weight () and standard length (). During the harvest season, samples of water, sediment, and fish (Bolti fish, Oreochromis niloticus, Topara fish, Chelon ramada (n = 3/pond)) samples were collected from the selected ponds and brought to the marine biology lab for further analysis.
HMs Levels Investigations
Water, sediment and fish tissues (spleen, muscle, liver, intestines, and gills) samples were prepared, digested and diluted in detail by Abbas et al. [2] according to AOAC [1]. However, the contents of Lead (Pb), Copper (Cu), Iron (Fe), Cadmium (Cd), Manganese (Mn), and Zinc (Zn) have been measured in the diluted solutions of sediment, water, and fish tissues, using an ICP-OES instrument (inductivelycoupledplasmaopticalemissionspectrometer, 4300 DV, Perkin-Elmer, Shelton, USA). Moreover, HMC in water is expressed in ppb (µg/L), while HMC in sediments and tissues is expressed in ppm dw-b (µg/g, dw-b) on a dry weight basis.
Metal Pollution Index (HMC-MPI)
The contamination levels of HMC in water, sediment, and in the tissues of Botti, O. niloticus and Topara, C. ramada were assessed using the HMC-MPI [12, 32] to measure the contamination degree in sediment, water, and in the studied fish tissues. The HMC-MPI was estimated using the following equation:
The contamination level is safe degree when the MPI-HMC value is less than 1, the MPI-HMC is between 1.0 and 2.0, conditions are categorized as slightly contaminated, 2.0 to 3.0, moderately to severely contaminated, 3.0 to 5.0, severely contaminated, and > 10 heavily contaminated.
Haematological, Biochemical, and Immunological Investigations
Regarding the evaluation of the several blood variables, sampling was performed. Haematology samples were obtained from the caudal vein of five fish that were anesthetized with clove oil (50 μL/L) using a syringe filled with EDTA blood, an anticoagulant. However, the biochemical and immunological samples were collected without the use of anticoagulants, centrifugation (3000/15 min.) at room temperature to obtain serum, and then stored at freezing point (-20 °C) until analysis. Fish haematological parameters (packed cell volume, white blood cellsred blood cells, blood cell indices, and haemoglobin levels,) were measured in EDTA blood samples according to Brown, [15], Van Kampen and Zijlstra [71], Dacie and Lewis [16]. Fish biochemical parameters (total protein (TP) and albumin (ALB) and activities of alanine and aspartate aminotransferase) were detected in blood-serum samples according to Henry [31], Reitman and Frankel [53], globulin level (GLO) was obtained by subtracting the ALB level from the TP level. Fish immune parameters (lysozyme activity, total immunoglobulin and complement C3 activities) in blood-serum samples were measured as described by Ellis [24], Siwicki and Anderson [59], and Tang et al. [62].
Health Risk Assessment (HRA)
We employed a technique established by the USEPA (2018) to evaluate the risk to human health of HMC consumed by ingestion of the muscle of the investigated fish. The estimated daily intake (EDI-HMC), non-carcinogenic (THQ-HMC and HI-HMC indexes) and carcinogenic indexes (CR-HMC) of HMC were all performed by detecting the HMCs in the Bolti and Topara muscle from the studied ponds (Table 1S).
Statistical Evaluations
Statistics program; software, Version 22; SPSS was used to perform the statistical evaluations. The results were tested for the significant differences using a one-way analysis of variance and where significant variations were found at P < 0.05, post hoc Tukey tests were used, this enabled us to detect the significant variations at P < 0.05 between the HMC in fish tissues. To further investigate the statistical differences between the fish supplies (KES and ES fishponds) of individual HMC, the T-test (independent sample) was used. However, the Pearson correlation coefficient was calculated to investigate the relationship between all HMCs in water, sediment, and the examined fish.
Results and Discussion
HMCs Estimation of Water and Sediment
HMC in the water and sediments of KES and ES fishponds are represented in Table 1. Concerning the metal levels, the sequencing of results reported at P < 0.05 as Fe (320.67 ± 3.66 ppb) > Zn(68.49 ± 1.23 ppb) > Cu(37.02 ± 1.02 ppb) > Mn (24.99 ± 1.22 ppb) > Pb (10.72 ± 0.89 ppb) > Cd (2.94 ± 0.35 ppb) in KES fishponds water, while it was Fe (416.00 ± 7.65 ppb) > Zn (110.05 ± 1.87 ppb) > Mn (49.79 ± 0.97 ppb) > Cu (39.44 ± 0.98 ppb) > Pb (15.40 ± 0.79 ppb) > Cd (5.10 ± 0.52 ppb) in ES fishponds water. However, in the sediment, HMCs showed significant differences (P < 0.05) as Fe (21312 ± 654–60332 ± 214 ppm dw-b) > Mn (846.33 ± 55.51–1058 ± 35.65 ppm dw-b) > Zn (58.33 ± 1.68–160.00 ± 1.19 ppm dw-b) > Cu (18.49 ± 5.32–53.72 ± 2.66 ppm dw-b) > Pb (10.56 ± 1.36–18.06 ± 1.32 ppm dw-b) > Cd (2.31 ± 0.17–2.76 ± 0.41 ppm dw-b) in both sites. These findings were supported by El-Batrawy et al. [22] who reported that the order of the metals exhibited the highest levels for Fe and the lowest was Cd in Burullus Lake water and in water farms [50]. Concerning the pond site, the HMCs in water and sediment samples were significantly higher in ES fishponds compared to KES fishponds. In comparison with the permissible limit [75], the studied essential HMCs in the water of ES and KES fishponds were lower than the permissible limit. However, non-essential HMC, Lead levels in ES and KES fishponds water and Cd in ES fishponds water were higher than the recommended limit. On the other hand, the studied essential HMC in the sediment of ES and KES fishponds was higher than in the upper continental crust, except for Iron in ES sediment. However, non-essential HMC, Cd levels in ES and KES fishponds sediment and Pb in KES fishponds sediment were higher than the upper continental crust [56].
HMCs Estimation of Cultured Fish
Metal levels are important markers of the health of fish and their surrounding environment [47]. Table 2 shows the distribution of HMC in the tissues of Bolti and Topara fish from the ES and KES fishponds. Concerning the cultured species, Bolti fish tissues in the examined ponds had significantly higher Fe, Cu, Pb, and Cd contents than Topara tissues. However, the Zn content in Bolti tissues was significantly higher than in Topara tissues, apart from muscle, which was significantly lower in Bolti tissues than Topara tissues. Additionally, the Mn content in Bolti tissues was significantly higher than that of Topara tissues, except for KES gills. However, the HMC of Bolti fish was higher than that of Topara fish in each of the studied ponds. HMC levels in different fish species vary according to their environmental requirements, physiological activity, and feeding habits [74].
Concerning the pond site, Bolti and Topara fish cultivated together had significantly higher contents of Zn, Fe, Cu, and Pb levels in ES fishponds than in KES fishponds (T-test, p < 0.05). However, the Cd content of Bolti and Topara fish was significantly lower in KES fishponds than in ES fishponds, except for Bolti fish liver, which was non-significant. Additionally, Mn content in Bolti fish in ES fishponds was significantly higher than in KES fishponds, whereas Mn content in Topara fish in ES fishponds was significantly lower than in KES fishponds (T-test, p < 0.05). The water source of the studied ponds is agricultural drainage water. However, the ES province included a large number of anthropogenic activities and industrial wastes that were discharged into the canals and drains than in KES province. Thus, cultured species in ES fishponds located in ES province with higher polluted levels of HMC than those in KES fishponds. Concerning the tissue sequence in each HMC, the Bolti tissues in both ponds are arranged in the following order: gills > liver > spleen > intestines > muscle for Zn and Fe; liver > spleen > gills > intestines > muscle for Pb and Cd; gills > liver > intestines > spleen > muscle for Cu; and liver > intestines > gills > spleen > muscle for Mn. However, in Topara tissues, the arrangement of HMC was gills > liver > spleen > intestines > muscle for Cu, Zn, and Fe; gills > intestines > liver > spleen > muscle for Mn; and gills > spleen > liver > intestines for Cd in both ponds. On the other hand, lead in Topara tissues has the following sequence: gills > spleen > liver > intestines in KES fishponds, and gills > spleen > liver > intestines > muscle in ES fishponds. Furthermore, the studied essential HMC (except Mn) of Bolti and Topara fish accumulated at the highest levels in the gills and liver. However, non-essential HMC detected the maximal levels in both the liver and spleen of Bolti fish and in both the gills and spleen of Topara fish. The HMC in an organism's tissues depends on several factors, including its species, sex, age, level of exposure to pollution, and water quality [40]. Fish kidneys, liver, and gills are examples of metabolically active organs that accumulate more HMC than muscle and skin, which are examples of metabolically fewer active organs [11, 42]. Moreover, the minimal levels of studied essential and non-essential HMC were recorded in muscles, and they also stored the least amount of HMC [55]. These findings are supported by Badr et al. [14] whom revealed that the HMC in tissues of Tilapia fish recorded the maximal levels in the liver followed by fish gill and reached the minimal level in the muscle [50]. The higher level of HMC accumulation detected in fish livers provides a useful biomarker of HMC water pollution [21], as a source of metal metabolic processes [83] the natural presence of metallic-binding proteins in hepatocyte tissues includes metallothioneins. This finding was widely reported in several fish species [3, 10, 57, 60, 65, 67].
Concerning the HMCs, the HMCs in the Bolti tissues of the studied ponds were arranged as: Fe > Zn > Cu > Pb > Mn > Cd for the liver, gills, spleen, and intestines, while it was Fe > Cu > Zn > Pb > Mn > Cd for muscle. However, there was abundance in the Topara tissues of the studied ponds as Fe > Zn > Cu > Pb > Mn > Cd for the liver, gills, and spleen, while it was Fe > Zn > Cu > Mn > Pb > Cd for the intestines. Otherwise, the Topara muscle has these arrangements: Fe > Zn > Cu > Mn > Pb in ES fishponds and Fe > Zn > Cu > Mn in KES fishponds. The accumulation of Iron in tissues of the cultivated fish increased more than the accumulation of other HMCS. These may be associated with the elevation of total dissolved Iron in the fishpond water and lakes, which could improve the free Iron content and hence HMC absorption by various organs. These observations are in agreement with Tayel et al. [63] and Al-Halani et al. [10] and Radwan et al. [50]. Furthermore, Cu, Fe, and Zn in Bolti and Topara fish exhibited higher essential metal content in most cases; this can be explained by the fact that these metals are essential for many biological processes and are consequently included in fish feed [58], suggesting that cultivated fish have higher levels of essential HMC [25, 81]. The maximal levels of toxic HMCs (Pb and Cd) in Bolti and Topara fish had, suggesting this may be due to their shortened lifespan and lower exposure levels (within 180 days) [45]. Furthermore, Fallah et al. [25], Kim et al. [36] Yildiz [79], and Simukoko et al. [58] revealed that diets with animal protein sources had much higher concentrations of HMC, especially those that are essential for human health such as Zinc, Iron, and Copper. The essential HMCs such as Zinc, Iron, Copper, and Manganese are expressed at higher levels due to their biological roles in comparison with the toxic HMCs, Cadmium and Lead, which are biologically insignificant and present at much lower levels in fish tissues. Numerous scholars brought up comparable circumstances [10, 20, 64, 66, 68].
In general, HMCs in the tissues of Bolti fish cultivated in KES fishponds exhibited the maximal level in the gills for Iron metal (111 ± 2.32 ppm dw-b) and the minimal level recorded in muscle for Cd metal (0.08 ± 0.001 ppm dw-b) while HMCs in the tissues of Topara fish fluctuated between 70.3 ± 2.18 ppm dw-b in the gills for Iron and 0.09 ± 0.001 ppm dw-b in the intestine for Cd metal. However, in KES fishponds, HMCs in the tissues of Bolti fish revealed the maximal value in the gills for Iron metal (132.3 ± 2.86 ppm dw-b) and the minimal value recorded in muscle for Cd metal (0.10 ± 0.003 ppm dw-b) while HMCs in the tissues of Topara fish varied from 77.65 ± 2.04 ppm dw-b in the gills for Iron to 0.18 ± 0.01 ppm dw-b in the muscle for Mn metal. According to the permissible limit [76], Zn, concentrations in tissues of two fish cultivated in both studied ponds were lower than the permissible limit of 40 ppm WHO-FAO [76] except in the liver, gills of Bolti fish of both studied ponds and gills of Topara fish cultured in ES fishponds which were higher than the permissible limit. However, Iron levels were decline than the permissible limit (100 ppm) in all tissues of the two cultivated fish except the liver and gills of Bolti fish which were higher than the permissible limit WHO-FAO [76]. Moreover, Cu, concentrations in the muscle, liver, intestines, and spleen of Bolti and Topara fish inhabiting ES and KES fishponds were lower than the permissible limit of 30 ppm while it was higher in gills WHO-FAO [76]. Except for the liver and spleen of Bolti fish, Lead concentrations in the two cultivated fish tissues were lower than the permissible limit of 2 ppm according to the World Health Organization WHO-FAO [76]. Furthermore, Cd and Mn, concentrations in all tissues of Bolti and Topara fish inhabiting ES and KES fishponds declined than the allowed limit (1 ppm) according to the World Health Organization WHO-FAO [76].
Pearson Correlation Coefficients
Table 3 displays the findings of a hierarchical cluster analysis of the water, sediment, and fishponds using six HMC Pearson correlation coefficients. High-positive relationships between HMCs are considered to have shared origins, whereas extremely negative relationships between HMCs are considered to have different origins [8]. However, correlations between HMCs can reveal information about their origin and their association. A significant positive correlation was observed between the following metals, implying that they may have originated from similar sources: Cu, Fe, Pb, and Zn for KES fishponds water; Cu, Fe, Mn, Cd, and Pb for ES fishponds water; Cu, Fe, Mn, and Pb for the sediment of the studied ponds; Cd for KES fishponds Bolti samples; Cu and Mn for ES fishponds Bolti; Cd and Pb for KES fishponds Bolti; Cu and Mn for ES fishponds Bolti; Cd and Pb for KES fishponds Topara samples; and Mn for ES fishponds Topara samples. The relationships between the different HMCs may stem from the similarities in the cumulative behaviour of the different HMCs in fish and their interactions [54]. According to Kumar et al. [37], the significant relationships between metals may be due to a shared origin for their occurrence and suggest similar biogeochemical processes for subsequent accumulation in fish muscle. In addition, the six HMCs of the water, sediment, and tissues of fishponds were separated into two clusters: Cu, Fe, Pb, and Zn; Cd and Mn for water and Bolti samples; Cu, Zn, and Cd; Fe, Mn, and Pb for sediment samples; and Cu, Fe, and Zn; Mn, Cd, and Pb in the case of Topara samples. However, in ES fishponds samples, it was also divided into two clusters: Cu, Fe, Mn, and Zn; Cd and Pb for Topara and Bolti samples; Zn; Cu, Fe, Mn, Cd, and Pb for water; and Zn-Cd; Cu, Fe, Mn, and Pb for sediment samples from ES fishponds.
Bio-Accumulation and Bio-Sedimentation Factors
Bio-accumulation (BAF-HMC) and bio-sedimentation factors (BSF-HMC) of HMC in the Topara and Bolti fish inhabiting KES and ES fishponds are shown in Fig. 2. The positive BAF-HMC values reported that the cultivated fish could derive HMCs from water [3]. The highest BAF-HMC values were observed for BAF-Cu, which ranged from 405 to 893, whereas the lowest BAF-Mn values were observed, with BAF-HMC values ranging from 3.6 to 34. The maximum values of BAF-HMC were recorded in Bolti tissues, and the lowest were observed in Topara tissues. However, it was higher in the tissues of fish inhabiting ES fishponds than in KES fishponds. The BAF-HMC values in Topara tissues exhibited their maximum level in the gills, and the minimum was observed in the muscle. However, in the Bolti fish, the highest BAF-HMC values were recorded in the gills for BAF-Zn, BAF-Fe, and BAF-Cu and in the liver for BAF-Pb, BAF-Cd, and BAF-Mn, while the lowest values were observed in the muscle. The diversity of biochemical and physiological characteristics of organs as well as the level of environmental contamination affect BAF-HMC values [70]. The order of fishpond water BAF-HMC was BAF-Fe > BAF- Zn > BAF-Cu > BAF-Mn > BAF-Pb > BAF-Cd, while the order of BAF-HMC was BAF-Cu > BAF-Zn > BAF-Fe > BAF-Cd > BAF-Pb > BAF-Mn in the Topara fish and BAF-Cu > BAF-Zn > BAF-Fe > BAF-Pb > BAF-Cd > BAF- Mn in the Bolti fish. This suggests that a variety of parameters, including environmental factors, the level and chemical structure of HMC, pollution degree, farm environments, age, species, feeding patterns, and metabolic rate, affects the bioavailability of HMC by fish [5, 19, 43].
Regarding the BAF-HMC values for Bolti samples, BAF-Cu in all tissues is of moderate concern (250 > BAF-HMC 1000), and BAF-Cd, BAF-Manganese, and BAF-Lead are of low concern (BAF-HMC 250) for all studied tissues. Except for muscle, where BAF-Zn was of medium concern (250 > BAF-HMC 1000), BAF-Zn is present in all tissues. While spleen, muscle, and liver Fe were of low concern (BAF-HMC 250), gills and liver BAF-Fe were of moderate concern (250 > BAF-HMC < 1000). However, in Topara fish, BAF-Cd, BAF-Mn, BAF-Fe, and BAF-Pb are of low concern (BAF-HMC < 250 > BAF-HMC < 1000 > BAF-HMC < 1000) except in muscle. The BAF-HMC provides details on the accumulation of specific metals in tissues of fish. The overall findings revealed that for almost every HMCs, metals accumulation in muscle, liver, gills, intestines, and spleen decreased in Topara fish compared to Bolti fish when comparing the BAF-HMC values of the two species. BAF-HMC changes from the environment to fish tissue depending on chemical category, organ metabolite properties, and degree of environmental contamination [13, 46, 82].
The BSF-HMC, on the other hand, measures the proportion of HMCs in sediment to those in tissues [4]. According to their BSF-HMC values, tissues are categorised into three groups: macro-concentrators (BSF-HMC values > 2), micro-concentrators (BSF-HMC values 1 to 2), and de-concentrators of metals (BSF-HMC values 1) [84]. Based on the above, the Bolti and Topara tissues from ES and KES fishponds showed that BSF-HMC of muscle, liver, gills, intestines, and spleen are de-concentrators (BSF-HMC < 1) for all metals and release the metals in sediment except Cu, tissues are micro-concentrator (1 < BSF-HMC < 2). This means that there is almost no BSF-HMC in studied fish tissues. Additionally, Bolti tissue in the study ponds had the highest BSF-HMC value, whereas Topara tissue had the lowest value. However, it was higher in the tissues of fish inhabiting ES fishponds than in KES fishponds. Topara gills had the highest BSF-HMC value of any tissue, while the muscle had the lowest value. In contrast, in the Bolti fish, the liver showed the highest BSF-HMC values for Pb, Cd, and Mn, and the gills showed the lowest values for Zn, Fe, and Cu. In general, the BAF-HMC value appears to be higher than the BSF-HMC value.
Metal Pollution Index (MPI-HMC)
The high MPI-HMC score indicates that the fish species has significant cumulative HMC accumulations [49] and poses a potential risk to public health is fish consumption with a high MPI-HMC value [5]. Figure 3 displays the presence of MPI-HMC in the samples of water, sediment, and tissues (Bolti and Topara fish) from the ES and KES fishponds. The MPI-HMC values of water, and sediment were over 10, this suggested the water and sediment of the different fishponds were heavily contaminated with heavy metal levels and it was higher in ES fishponds than in KES fishponds. Except for Topara muscle, MPI-HMC samples measured higher levels of metal pollution for all samples from ES fishponds compared to the KES fishponds. Therefore, this result shows that ES fishponds are more polluted by the investigated metals than KES fishponds.
Haematological, Immunological, and Biochemical Alterations
Blood variables are widely employed to examine fish well-being due to the fact they are easily determined and strongly related to environmental and physiological fish behavior [17]. Haematological indices are the most critical parameters used as indicators of the health of fish [48]. Haematological, immunological, and biochemical examination of Bolti and Topara fish are mentioned in Table 4. The heamatology of Bolti and Topara cultured in ES fishponds detected significantly decrease (p < 0.05) in Hb, MCHC, RBC count, PCV, and MCH compared with those fish cultured in KES fishponds. Conversely, WBC count and MCV showed significantly increase (P < 0.05) in fish cultured in ES fishponds in comparison to these fish cultured in KES fishponds. Insufficient levels of the studied haematological markers could indicate hemodilution or anaemia attributed to metabolism failure [72]. In addition, serum biochemical indicators demonstrated a significant increase (P < 0.05) in ALAT and ASAT activity and a significant decrease in total protein, albumin, and globulin in cultivated fish in ES fishponds compared to these fish cultured in KES fishponds. The decrease in protein levels has been linked to physiological deficits, malnutrition, infections, and haemodilution, which decreased absorption of nutrients [18]. Also, protein deficiency in fish can be caused by changes in water quality due to waste from many causes, such as contamination in industrial and agricultural water supplies [77]. However, immunological assay declared a significant decrease (P < 0.05) in total Ig (mg/mL), complement C3 (mg/mL), and lysozyme (U/mL) in Bolti and Topara samples cultured in ES fishponds compared to fish cultured in KES fishponds. These results correspond to the HMC and their contamination degree and confirm that the HMC contamination levels affected the physiological and immune status of Bolti and Topara fish cultured in ES fishponds compared to cultured fish in KES fishponds.
Human Health Hazard
Estimated daily exposure to HMC by consumption of fish high in HMC was required to avoid any adverse impacts on human health over the duration of a person's lifetime [51]. The allowable quantities of HMC were established using the EDI-HMC [35]. EDI-HMC values (mg kg-1 day-1) in the Bolti and Topara muscles cultured in ES and KES fishponds are shown in Fig. 4. Regarding species, the EDI-HMC value in the Bolti muscle cultured in the studied ponds was higher than that in the Topara muscle, which implied that the Bolti fish establish a high rate of exposure for customers (children and adults) through HMC consumption. The EDI-Fe, EDI-Zn, EDI-Cu, EDI-Ni, EDI-Cd, and EDI-Pb values in the Bolti and Topara muscles from the different fishponds were less than the permissible tolerable daily intake, PTDI, based on limits according to FAO-WHO guidelines. The PTDI values of Fe, Zn, Cu, Mn, Cd, and Pb are 50, 70, 50, 4E-02, 3E-03, and 3E-02 mg/kg/day, respectively [27, 33]. The EDI-HMC value was higher in fish cultured in ES fishponds in comparison to samples cultured in KES fishponds, which implied that the fish of ES fishponds exert the highest exposure in both children and adults through the HMC intake.
The THQ-HMC allowable threshold limit is 1.00. The degree of exposure was still lower than the advised dosage when THQ-HMC was below the unit limit; hence, exposure to HMC won't have a negative impact on lifetime intake [78]. However, the target hazard quotient (THQ-HMC) values for Mn, Iron, Cd, Copper, Lead and Zinc in the muscles of the studied species cultured in KES and ES fishponds are illustrated in Fig. 4. The THQ-HMC values in the muscles of the studied species were all below 1. Additionally, under USEPA [69], the THQ-HMC estimate for each HMC is added up and represented as a hazard index, HI-HMC, to measure the total possible non-carcinogenic effects caused by many metals. The THQ-HMC data were used to calculate the risk index (HI-HMC) for both adults and children consuming the two species; if the HI-HML value was > 1, the effect on consumers had an adverse effect, as suggested by Lei et al. [38]. The HI-HMC values in the Bolti and Topara muscles of the two ponds were both less than one, indicating no non-carcinogenic risk for human intake occurred (Fig. 5).
The non-essential HMC Pb and Cd may increase the risk of human cancer (CR-HMC, [39]). Furthermore, the carcinogenic index (CR-HMC) values of Cadmium and Lead in the Bolti and Topara muscles of the two ponds were determined for eaters (adults and children), and the results are presented in Figs. 5. The CR-Pb values in Bolti and Topara muscles were less than 1E − 6 for both children and adult consumers, suggesting that the CR-Pb was safe [73]. However, Cd poses a cancer risk (CR-Cd) to children’s consumers of Bolti and Topara muscle in ES fishponds, as the CR-Cd values were higher than the acceptable limit value of 1E − 6 [35]. Generally, the non-cancer (THQ-HMC and HI-HMC) and cancer (CR-Pb and CR-Cd) risks of HMCs in muscles of cultivated Bolti and Topara fish were elevated in ES fishponds than in KES fishponds. This suggests that fish cultivated in ES fishponds are more polluted with HMCs than fish cultivated in KES fishponds. Regarding to previous literatures, children’s consumers were more prone to CR-HMC exposure, Ahmed et al. [7], Abtahi et al. [6], Noman et al. [44] and Raknuzzaman et al. [52]
Conclusion
The Zinc, Fe, Cu, Pb, Cd, and Mn concentrations were presented in varying levels in tissues, sediment and water samples, with the accumulation rate depending on the species (Bolti and Topara), the tissues (spleen, muscle, liver, intestines, and gills), and fishponds (ES and KES fishponds). The findings mentioned that it was a significantly difference (T-test, p < 0.05) between the studied fishponds on HMC, recorded in tissues, water, and sediment, with elevated concentration found in ES-fishponds than in KES-fishponds. It was revealed that in Topara and Bolti fish, the HMC contents in their muscles were lower than the acceptance limits. El-Sharkia ponds had higher levels of HMC distribution and accumulation in the sediment, water, and fish tissues than KES fishponds, indicating that ES fishponds have higher levels of pollutants in comparison to KES fishponds. This may be reflected in the differences between Kafr El-Sheikh and Sharkia fishponds in terms of the haematological, immunological, and biochemical changes that occur there, as well as in the results on the degree of HMC contamination in both fishponds. Children and adults were both exposed to HMC through the consumption of cultivated fish in different fishponds, but no notable adverse non-carcinogenic risks to the consumers were identified, as the estimated values of THQ-HMC and HI-HMC were < 1. Nonetheless, the CR-Pb values for both children and adult consumers were less than the allowed limit, meaning that the carcinogenic hazard caused by Pb was safe, while Cd poses a cancer risk (CR-Cd) to children’s consumers of Bolti and Topara muscle in ES fishponds, as the CR-Cd values were higher than the acceptable limit. These investigated areas (ES and KES) are crucial to the Egyptian aquaculture industries of Bolti and Topara. Future heavy metal contamination in fish farms should be minimised through regular monitoring and awareness-raising, thereby reducing the risk to public health. Therefore, to protect the Bolti and Topara aquaculture sectors in Egypt, more detailed studies are required with a focus on metals in fish feed, water quality, waste management, and handling processes.
Data Availability
The data sets in this study are available from the corresponding author on reasonable request.
References
A.O.A.C. Association of Official Analytical Chemists (2012) Official methods of analysis, 15th edn. Association of Official Analytical Chemists, Washington, DC, USA, p 478
Abbas MMMM, Afifi AM, Darweesh KF, El-Sharkawy MA, Farrag DMG, Radwan M (2023) Parasitological indicators, haemato-biochemical alternations, and environmental risks of heavy metals in cultivated and wild freshwater catfish, Egypt. Egypt J Aquatic Biol Fish 27(4):1085–1205. https://doi.org/10.21608/EJABF.2023.314426
Abbas MMM, Abd El-Aziz MAE, Kaddah MMY, Hassan AK, El-Naggar HA, Radwan M, El-Tabakh MAM, Afifi MA, Bashar MAE (2022) Bioaccumulation, Biosedimentation, and health hazards of elements in crayfish, procambarus clarkii from el-rahawi drain and el-qanatir in the river nile. Egypt Biol Trace Elem Res 1(6):3050–3059. https://doi.org/10.1007/s12011-022-03380-7
Abdallah MAM, Abdallah AMA (2008) Biomonitoring study of heavy metals in biota and sediments in the South Eastern coast of Mediterranean sea. Egypt Environ Monit Assess 146:139–145. https://doi.org/10.1007/s10661-007-0066-8
Abdel Ghani SA, Ibrahim MIA, Shreadah MA, El-Sayed AAM, Aly-Eldeen MA (2023) Ecological risk assessment of selected contaminants in seawater, sediment and some fish species from Alexandria beaches, South-Eastern Mediterranean Sea, Egypt. Environ Nanotechnol Monit Manag 20:100873. https://doi.org/10.1016/j.enmm.2023.100873. (ISSN 2215-1532)
Abtahi M et al (2017) Heavy metals (As, Cr, Pb, Cd and Ni) concentrations in rice (Oryza sativa) from Iran and associated risk assessment: A systematic review. Toxin Rev 36:331–341
Ahmed ASS, Rahman M, Sultana S, Babu SMOF, Sarker MSI (2019) Bioaccumulation and heavy metal concentration in tissues of some commercial fishes from the Meghna River Estuary in Bangladesh and human health implications. Mar Pollut Bull 145:436–447. https://doi.org/10.1016/j.marpolbul.2019.06.035
Al-Alimi A, Alhudify NS (2016) Assessment of heavy metals contamination and its ecological risk in the surface sediments of AlMukalla coast, Yemen. J Sci Eng Res 3:13–23 (https://www.semanticscholar.org/paper/Assessment-of-heavy-metals-contamination-and-its-in-Al-Alimi-Alhudify/1e70673b2626cd82199e90a66a0dc7e11652df68)
Alam M, Md Rohani F, Md Hossain S (2023) Heavy metals accumulation in some important fish species cultured in commercial fish farm of Natore, Bangladesh and possible health risk evaluation. Emerging Contaminants 9(4):100254. https://doi.org/10.1016/j.emcon.2023.100254
Al-Halani Ali A, Soliman Ashraf M, Shady Sherien HH (2021) The seasonal assessment of heavy metals bioaccumulation in European seabass (Dicentrarchus labrax) inhabiting damietta fishing harbor, Egypt. Egypt J Aquat Biol Fish 25(5):607–626 (https://ejabf.journals.ekb.eg/article_205025.html)
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J Chem 6730305. https://doi.org/10.1155/2019/6730305
Ali MM, Saiful Islam M, Towfiqul Islam AM, Bhuyan MS, Ahmed ASS, Rahman MZ, Rahman MM (2022) Toxic metal pollution and ecological risk assessment in water and sediment at ship breaking sites in the Bay of Bengal Coast, Bangladesh. Mar Pollut Bull 175:113274. https://doi.org/10.1016/j.marpolbul.2021.113274
Ayaş Z (2007) Trace element residues in eggshells of grey heron (Ardea cinerea) and black-crowned night heron (Nycticorax nycticorax) from Nallihan Bird Paradise, Ankara-Turkey. Ecotoxicology 16:347–352. https://doi.org/10.1007/s10646-007-0132-6
Badr AM, Mahana NA, Eissa A (2014) Assessment of Heavy Metal Levels in Water and Their Toxicity in Some Tissues of Nile Tilapia (Oreochromis niloticus) in River Nile Basin at Greater Cairo, Egypt. Global Veterinaria 13(4):432–443
Brown L (1993) Aquaculture for Veterinarians. Fish Husbandry and Medicine Pergamon Press Ltd, Oxfor. https://www.amazon.com/Aquaculture-Veterinarians-Fish-Husbandry-Medicine/dp/0080408354
Dacie J, Lewis S (1991) Reference Ranges and Normal Values. In Practical Haematology (New York: Churchill Livingstone), pp 9–17. https://doi.org/10.1016/B0-44-306660-4/50006-4
De Pedro N, Anton AG, Lopez-Patino MA, Martınez-Alvarez R, Delgado MJ (2005) Daily and seasonal variations in haematological and blood biochemical parameters in the tench, Tinca tinca Linnaeus, 1758. Aquac Res 36(12):1185–1196. https://doi.org/10.1111/j.1365-2109.2005.01338.x
Del Rio-Zaragoza OB, Fajer-Ávila EJ, Almazán-Rueda P, Abdo de la Parra MI (2011) Haematological characteristics of the spotted rose snapper Lutjanus guttatus (Steindachner, 1869) healthy and naturally infected by dactylogyrid monogeneans. Tissue Cell 43(3):137–142. https://doi.org/10.1016/j.tice.2011.01.002
Dhanakumar S, Solaraj G, Mohanraj R (2015) Heavy metal partitioning in sediments and bioaccumulation in commercial fish species of three major reservoirs of river Cauvery delta region, India. Ecotoxicol Environ Safety 113:145–151. https://doi.org/10.1016/j.ecoenv.2014.11.032
Dural M, Göksu MZL, Özak AA (2007) Investigation of heavy metal levels in economically important fish species captured from the Tuzla lagoon. Food Chem 102:415–421
Dural M, Genc E, Sangun M, Güner O (2011) Accumulation of some heavy metals in Hysterothylacium aduncum (Nematoda) and its host sea bream, Sparusaurata (Sparidae) from North-Eastern Mediterranean Sea (Iskenderun Bay). Environ Monit Assess. 174(1–4):147–155
El-Batrawy OA, El-Gammal MI, Mohamadein LI, Darwish DH, El-Moselhy KM (2018) Impact assessment of some heavy metals on tilapia fish, Oreochromis niloticus, in Burullus Lake, Egypt. J Basic Appl Zool 79:13. https://doi.org/10.1186/s41936-018-0028-4
El-Gaar DM, Abdelaziz GS, Abbas MMM, Anees FR, Genina ME, Ghannam HE, Talab AS (2022) Biochemical alterations of Nile tilapia fish in El-Manzala Lake as an indicator of pollution impacts. Egypt J Aquat Biol Fish 26(3):711–723. https://doi.org/10.21608/ejabf.2022.246093
Ellis AE (1990) Lysozyme assays. Techniques Fish Immunol 12(1):101–103 (https://scholar.google.com.eg/scholar?q=ellis,+a.e.+(1990).+lysozyme+assays.+techniques+in+fish+immunology.&hl=ar&as_sdt=0&as_vis=1&oi=scholart)
Fallah AA, Saei-Dehkordi SS, Nematollahi A, Jafari T (2011) Comparative study of heavy metal and trace element accumulation in edible tissues of farmed and wild rainbow trout (Oncorhynchus mykiss) using ICP-OES technique. Microchem J 98:275–9. https://doi.org/10.1016/j.microc.2011.02.007
FAO (2020) The State of World Fisheries and Aquaculture 2020. Sustainability in Action. Rome. https://www.fao.org/documents/card/en/c/ca9229en. https://doi.org/10.4060/ca9229en
FAO/WHO (2004) FAO/WHO Summary of Evaluations Performed by the Joint FAO/WHO Expert Committee on Food Additives (JECFA 1956e2003). Evaluation of Certain Contaminants in Food: Sixty-Fourth Report of the Joint
Farrag DMG, Azab AM, Alabssawy AN (2021) Histological variations and adaptability in some digestive organs of the thinlip grey mullet, Chelon ramada (Risso, 1827). Egypt J Aquat Biol Fish 25(1):241–256. https://doi.org/10.21608/EJABF.2021.138317, https://ejabf.journals.ekb.eg/article_138317.html. https://doi.org/10.21608/EJABF.2020.71233
Franco-Martínez L, Martínez-Subiela S, Cerón JJ, Tecles F, Eckersall PD, Oravcova K, Tvarijonaviciute A (2020) Biomarkers of health and welfare: A One Health perspective from the laboratory side. Res Vet Sci 128:299–307. https://doi.org/10.1016/j.rvsc.2019.12.012
Helal AM, Essa MA, Abdelaty BS, Elokaby MA (2020) The Effect of Pond Soil Types and Stocking Weight of Mugil capito Fingerlings Reared in Poly-culture System on Production Performance. Egypt J Aquat Biol Fish 4(1):431–441 (https://ejabf.journals.ekb.eg/article_71233.html)
Henry RJ (1964) Colorimetric determination of total protein. Clinical Chemistry. Harper and Row Publ., New York, USA, 181. https://scholar.google.com/scholar?q=Henry+R.J.+Colorimetric+determination+of+Total+Protein:+Clinical+Chemistry+1964+Harper+and+Row+New+York+181+https://journals.ekb.eg/article_61874_34fe04ef5e60601709ca99b123e7e639.pdf
Hossain, M.B., Tanjin, F., Rahman, M.S., Yu, J., Akhter, S., Noman, M.A., & Sun, J., (2022). Metals Bioaccumulation in 15 Commonly Consumed Fishes from the Lower Meghna River and Adjacent Areas of Bangladesh and Associated Human Health Hazards. Toxics, Mar 12; 10(3):139. DOI: https://doi.org/10.3390/toxics10030139
Ikem A, Ayodeji OJ, Wetzel J (2021) Human health risk assessment of selected metal(loid)s via crayfish (Faxonius virilis; Procambarus acutus acutus) consumption in Missouri. Heliyon 7(6):e07194. https://doi.org/10.1016/j.heliyon.2021.e07194
Jabeen F, Chaudhry AS (2010) Environmental impacts of anthropogenic activities on the mineral uptake in Oreochromis mossambicus from Indus River in Pakistan. Environ Monit Assess 166:641–651. https://doi.org/10.1007/s10661-009-1029-z (https://link.springer.com/content/pdf/10.1007/s10661-009-1029-z.pdf)
Keshavarzi B, Hassanaghaei M, Moore F, Mehr MR, Soltanian S, Lahijanzadeh AZ, Sorooshian A (2018) Heavy metal contamination and health risk assessment in three commercial fish species in the Persian Gulf. Mar Pollut Bull 129(1):245–252. https://doi.org/10.1016/j.marpolbul.2018.02.032
Kim HT, Loftus JP, Mann S, Wakshlag JJ (2018) Evaluation of arsenic, cadmium, lead and mercury contamination in over-the-counter available dry dog foods with different animal ingredients (Red Meat, Poultry, and Fish). Front Vet Sci 5:264. https://doi.org/10.3389/fvets.2018.00264
Kumar B, Mukherjee DPN, Kumar S, Mishra M, Prakash D, Singh SK, Sharma CS (2011) Bioaccumulation of heavy metals in muscle tissue of fishes from selected aquaculture ponds in east Kolkata wetlands. Ann Biol Res 2(5):125–134 (http://scholarsresearchlibrary.com/archive.html)
Lei M, Tie B-Q, Song Z-G, Liao B-H, Lepo JE, Huang Y-Z (2015) Heavy metal pollution and potential health risk assessment of white rice around mine areas in Hunan Province, China. Food Secur 7(1):45–54. https://doi.org/10.1007/s12571-014-0414-9
Li K, Ricker K, Tsai FC, Hsieh CJ, Osborne G, Sun M, Marder ME, Elmore S, Schmitz R, Sandy MS (2021) Estimated Cancer Risks Associated with Nitrosamine Contamination in Commonly Used Medications. Int J Environ Res Public Health 18(18):9465. https://doi.org/10.3390/ijerph18189465
Łuczy’nska J, Paszczyk B (2019) Health Risk Assessment of Heavy Metals and Lipid Quality Indexes in Freshwater Fish from Lakes of Warmia and Mazury Region, Poland. Int J Environ Res Public Health 16:3780
Maulu S, Hasimuna OJ, Haambiya LH, Monde C, Musuka CG, Makorwa TH, Munganga BP, Phiri KJ, Nsekanabo JD (2021) Climate Change Effects on Aquaculture Production: Sustainability Implications, Mitigation, and Adaptations. Front Sustain Food Syst 5:609097 (https://www.frontiersin.org/articles/10.3389/fsufs.2021.609097/full)
Maurya PK, Malik DS, Yadav KK, Kumar A, Kumar S, Kamyab H (2019) Bioaccumulation and potential sources of heavy metal contamination in fish species in River Ganga basin: Possible human health risks evaluation. Toxicol Rep 6:472–481. https://doi.org/10.1016/j.toxrep.2019.05.012
Moiseenko TI, Kudryavtseva LP (2001) Trace metal accumulation and fish pathologies in areas affected by mining and metallurgical enterprises in the Kola Region Russia. Environ Pollut 114:285–297. https://doi.org/10.1016/S0269-7491(00)00197-4
Noman MA, Feng W, Zhu G et al (2022) Bioaccumulation and potential human health risks of metals in commercially important fishes and shellfishes from Hangzhou Bay, China. Sci Rep 12:4634. https://doi.org/10.1038/s41598-022-08471-y
Oumar DA, Flibert G, Tidjani A, Rirabe N, Patcha M, Bakary T et al (2018) Risks assessments of heavy metals bioaccumulation in water and tilapia nilotica fish from maguite island of fitri lake. Curr J Appl Sci Technol 26:1–9. https://doi.org/10.9734/cjast/2018/39384
Ozmen M, Ayas Z, Güngördü A, Ekmekci GF, Yerli S (2008) Ecotoxicological assessment of water pollution in Sariyar Dam Lake, Turkey Ecotoxicol. Environ Saf 70:163–173
Padrilah SN, Shukor MYA, Yasid NA, Ahmad SA, Sabullah MK, Shamaan NA (2018) Toxicity effects of fish histopathology on copper accumulation. Pertanika J Trop Agric Sci 2018:519–540
Pradhan SC, Patra AK, Pal A (2014) Haematological and plasma chemistry of Indian major carp, Labeo rohita (Hamilton, 1822). J Appl Ichthyol 30(1):48–54. https://doi.org/10.1111/jai.12297
RabiulIslam GMM, Habib MR, Waid JL, Rahman MS, Kabir J, Akter S, Jolly YNN (2017) Heavy metal contamination of freshwater prawn (Macrobrachium rosenbergii) and prawn feed in Bangladesh: A market-based study to highlight probable health risks. Chemosphere 170:282–289. https://doi.org/10.1016/J.CHEMOSPHERE.2016.11.163
Radwan M, Abbas MMM, Afifi MAM, Mohammadein A, Al Malki JS (2022) Fish parasites and heavy metals relationship in wild and cultivated fish as potential health risk assessment in Egypt. Front Enviro. Sci 10:890039. https://doi.org/10.3389/fenvs.2022.890039
Rakib MRJ, Jolly YN, Enyoh CE, Khandaker MU, Hossain MB, Kther S, Alsubaie A, Almalki ASA, Bradley DA (2021) Levels and health risk assessment of heavy metals in dried fish consumed in Bangladesh. Sci Rep 11:14642. https://doi.org/10.1038/s41598-021-93989-w
Raknuzzaman M et al (2016) Trace metal contamination in commercial fish and crustaceans collected from coastal area of Bangladesh and health risk assessment. Environ Sci Pollut Res 23:17298–17310. https://doi.org/10.1007/s11356-016-6918-4
Reitman S, Frankel S (1957) Colorimetric determination of glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:53–56. https://doi.org/10.1093/ajcp/28.1.56
Rejomon G, Nair M, Joseph T (2010) Trace metal dynamics in fishes from the southwest coast of India. Environ Monit Assess 167(1–4):243–255. https://doi.org/10.1007/s10661-009-1046-y
Ronagh MT, Savari A, Papahn F, Hesni MA (2009) Bioaccumulation of heavy metals in Euryglossa orientalis from the Hendijan Seaport (Coastal of Persian Gulf). Iran J Biol Sci 9:272–275. https://doi.org/10.3923/jbs.2009.272.275
Rudnick RL, Gao S (2003) Composition of the continental crust. In: Rudnick RL (ed) The Crust, vol 3. Elsevier, pp 1–64
Shahjahan MD, Taslima K, Rahman MS, Al-Emran MD, Alam SI, Faggio C (2022) Effects of heavy metals on fish physiology – A review. Chemosphere 300:134519. https://doi.org/10.1016/j.chemosphere.2022.134519
Simukoko CK, Mwakalapa EB, Bwalya P, Muzandu K, Berg V, Mutoloki S, Polder A, Lyche JL (2022) Assessment of heavy metals in wild and farmed tilapia (Oreochromis niloticus) on Lake Kariba, Zambia: implications for human and fish health. Food Additives & Contaminants: Part A 39(1):74–91. https://doi.org/10.1080/19440049.2021.1975830
Siwicki AK, Anderson DP (1993) Nonspecific defense mechanisms assay in fish. II. Potential killing activity of neutrophils and monocytes, lysozyme activity in serum and organs and total immunoglobulin (Ig) level in serum. in Fish Diseases Diagnosis and Prevention Methods. FAO-project GCP/INT/526/JPN, pp 105–111. https://pubs.usgs.gov/publication/95381
Sobihah NNA, Zaharin A, Nizam MK, Juen LL, Woong KK (2018) Bioaccumulation of heavy metals in maricultured fish, Lates calcarifer (Barramudi), Lutjanus campechanus (red snapper) and Lutjanus griseus (grey snapper), Chemosphere, Volume 197. ISSN 318–324:0045–6535. https://doi.org/10.1016/j.chemosphere.2017.12.187
Soltan M, Mohamed H, Fayza A, Abdel-Rahman Kh (2016) Agricultural drainage water as a source of water for fish farming in Egypt. Ecol Evol Biol 1(3):68–75. https://doi.org/10.11648/j.eeb.20160103.15
Tang HG, Wu TX, Zhao ZY, Pan XD (2008) Effects of fish protein hydrolysate on growth performance and humoral immune response in large yellow croaker (Pseudosciaena crocea R.). J Zhejiang University-Sci B (Biomed Biotechnol) 9:684–690. https://doi.org/10.1631/jzus.B0820088
Tayel SI, Yacoub AM, Mahmoud SA (2008) Histopathological and haematological responses to freshwater pollution in the Nile catfish Clarias gariepinus. J Egypt Acad Soc Environ Develop 9(4):43–60
Tepe Y, Türkmen M, Türkmen A (2008) Assessment of heavy metals in two commercial fish species of four Turkish seas. Environ Monit Assess 146:277–284
Tepe Y, Türkmen A, Türkmen M (2017) Comparison of heavy metal accumulation in tissues of economically valuable fish species from two nearby lagoons in Mediterranean coastal area. Indian J Geo Mar Sci 46(7):1333–1338
Türkmen M, Türkmen A, Tepe Y, Ateş A, Gökkuş K (2008) Determination of metal contaminations in sea foods from Marmara, Aegean and Mediterranean seas: twelve fish species. Food Chem 108:794–800
Tyokumbur ET (2016) Bioaccumulation of heavy metals in fish species Sarotherodon melan other one from Alaro stream ecosystem in Ibadan. New York Sci J 9:83–88
Uluozlu OD, Tuzen M, Mendil D, Soylak M (2007) Trace metal content in nine species of fish from the Black and Aegean Seas. Turkey Food Chem 104:835–840
USEPA (2011) Risk Assessment Guidance for Superfund. Volume I: (Part A: Human Health Evaluation Manual; Part E, Supplemental Guidance for Dermal Risk Assessment; Part F, Supplemental Guidance for Inhalation Risk Assessment). EPA US Environmental Protection Agency, Washington, DC. https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-part, https://www.epa.gov/sites/default/files/2015-09/documents/rags_a.pdf
Uysal K, Köse E, Bülbül M, Dönmez M, Erdoğan Y, Koyun M, Ömeroğlu Ç, Özmal F (2009) The comparison of heavy metal accumulation ratios of some fish species in Enne Dame Lake (Kütahya/Turkey)Environ. Monit Assess 157:355–362
Van Kampen EJ, Zijlstra WG (1983) Spectrophotometry of hemoglobin and hemoglobin derivatives. Adv Clin Chem 23:199–257. https://doi.org/10.1016/S0065-2423(08)60401-1
Vivanco-Aranda M, Del Ríozaragoza OB, Lechuga-Sandoval CE, Viana MT, Rombenso AN (2018) Health response in yellowtail Seriola dorsalis exposed to an Amyloodinium ocellatum outbreak. Cienc Mar 44(4):44–65. https://doi.org/10.7773/cm.v44i4.2858
Wang X, Wu J, Yu B, Dong KF, Ma D, Xiao G, Zhang C (2020) Heavy metals in aquatic products and the health risk assessment to population in China. Environ Sci Pollut Res 27(18):22708–22719. https://doi.org/10.1007/s11356-020-08685-5
Weber P, Behr ER, Knorr CDL, Vendruscolo DS, Flores EMM, Dressler VL, Baldisserotto B (2013) Metals in the water, sediment, and tissues of two fish species from different trophic levels in a subtropical Brazilian river. Microchem J 106:61–66. https://doi.org/10.1016/j.microc.2012.05.004
WHO, World Health Organization (2011) Guidelines for drinking water quality. WHO Publications, Geneva, Switzerland, p 564. https://apps.who.int/iris/bitstream/handle/10665/336875/WHO-HSE-PHE-EPE-11.01.07-eng.pdf
WHO-FAO (1989) National research council recommended dietary 626 allowances (10th ed.) National Academy Press, Washington, DC. USA https://doi.org/10.17226/1349
Yacoub AM, Gad NS (2012) Accumulation of some heavy metals and biochemical alterations in muscles of Oreochromis niloticus from the River Nile in Upper Egypt. Intern J of enviro Scie and Engin 3:1–10
Yi Y, Yang Z, Zhang S (2011) Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ Pollut 159:2575–2585. https://doi.org/10.1016/j.envpol.2011.06.011
Yildiz M (2008) Mineral composition in fillets of sea bass (Dicentrarchus labrax) and sea bream (Sparus aurata): A comparison of cultured and wild fish. J Appl Ichthyol 24:589–594. https://doi.org/10.1111/j.1439-0426.2008.01097.x
Yipel M, Tekeli IO, Dikmen B (2021) Distribution and Ecotoxicological Risk Assessment of Heavy Metals in Streams of Amanos Mountains from Southern Turkey. Bull Environ Contam Toxicol 107:895–903. https://doi.org/10.1007/s00128-021-03316-2
Yipel M, Türk E, Tekeli IO, Oğuz H (2016) Heavy Metal levels in Farmed and Wild Fishes of Aegean Sea and Assessment of Potential Risks to Human Health. Kafkas Universitesi Veteriner Fakultesi Dergisi 22(6):889–894. https://doi.org/10.9775/kvfd.2016.15576
Younis AM, Amin HF, Alkaladi A, Mosleh YYI (2015) Bioaccumulation of heavy metals in fish, squids and crustaceans from the Red Sea, Jeddah Coast, Saudi Arabia. Open J Mar Sci 5:369
Zhao F-JT, Song ZJ-J, Huang X-Y, Wang P (2022) Toxic metals and metalloids: Uptake, transport, detoxification, phytoremediation, and crop improvement for safer food. Mol Plant 15(1):27–44. https://doi.org/10.1016/j.molp.2021.09.016. (ISSN 1674-2052)
Ziyaadini M, Yousefiyanpour Z, Ghasemzadeh J, Zahedi M (2017) Biota-sediment accumulation factor and concentration of heavy metals (Hg, Cd, As, Ni, Pb and Cu) in sediments and tissues of Chiton lamyi (Mollusca: Polyplacophora: Chitonidae) in Chabahar Bay, Iran. Iran J Fish Sci 16(4):1123–1134 (https://www.researchgate.net/publication/320539186_Biota-sediment_accumulation_factor_and_concentration_of_heavy_metals_Hg_Cd_As_Ni_Pb_and_Cu_in_sediments_and_tissues_of_Chiton_lamyi_Mollusca_Polyplacophora_Chitonidae_in_Chabahar_Bay_Iran)
Acknowledgements
Thanks are indebted to ALLAH, always and foremost, for his mercy guiding and helping. The authors’ gratefully acknowledge the Department of Zoology Depart., Faculty of Science, Al-Azhar University, Cairo, Egypt for providing the necessary support. Finally, we thank the anonymous reviewers of this article for their careful work and constructive suggestions.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Mahmoud Mahrous M. Abbas designed the research idea, Methodology, Original draft writing, Statics-Formal analysis.
Salah M. EL-Sharkawy Methodology, Writing – review and editing Visualization.
Hassan R. Mohamed Writing editing, Formal-analysis, figuration, and Tabulation.
Bassem E. Elaraby Figuration, and Tabulation Statics-formal analysis, review and editing.
Walaa M. Shaban Language editing, review and revised statistical analysis.
Metwally G. Metwally Figuration and Tabulation, Methodology.
Diaa M. G. Farrag Sampling Methodology, Conceptualization, Editing.
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent To Participate
All applicable guidelines (international, national, and/or institutional) for the care of fish were closely followed by the authors during the experimental works.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Six HMCs were monitored in Bolti and Topara fish farmed at Egyptian fishponds.
• Levels of HMC in muscular tissue were lower than those in the liver.
• HMCs in edible flesh were below the corresponding permissible limits.
• Immunological alterations were significant with HMC pollution.
• Bolti and Topara muscle suggest a safe non-cancer risk to human health.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abbas, M.M.M., EL-Sharkawy, S.M., Mohamed, H.R. et al. Heavy Metals Assessment and Health Risk to Consumers of Two Commercial Fish Species from Polyculture Fishponds in El-Sharkia and Kafr El-Sheikh, Egypt: Physiological and Biochemical Study. Biol Trace Elem Res (2023). https://doi.org/10.1007/s12011-023-04007-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-023-04007-1