Abstract
Inorganic fluoride is a geogenic and anthropogenic contaminant widely distributed in the environment and commonly identified in contaminated groundwater. There is limited information on the effect of fluoride exposure on pregnancy. The aim of this study was to evaluate possible placental alterations of fluoride exposure in a rat model simulating preconception and pregnancy exposure conditions in endemic areas. Fluoride exposure was administered orally to foetuses of dams exposed to 2.5 and 5 mg fluoride/kg/d. Foetal weight, height, foetal/placental weight ratio, placental zone thickness, levels of malondialdehyde (MDA) and vascular endothelial growth factor-A (VEGF-A) and vascular density in placental tissue were evaluated. The results showed a nonlinear relationship between these outcomes and the dose of fluoride exposure. In addition, a significant increase in the fluoride concentration in placental tissue was observed. The group that was exposed to 2.5 mg fluoride/kg/d had a greater increase in both MDA levels and VEGF-A levels than the higher dose group. A significant increase in the thickness of the placental zones and a decrease in the vascular density of the labyrinth zone area were also observed in the fluoride-exposed groups. In conclusion, the data obtained demonstrate that fluoride exposure results in morpho-structural alterations in the placenta and that non-monotonic changes in MDA, VEGF-A levels and placental foetal weight ratio were at environmentally relevant concentrations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inorganic fluoride is ubiquitous in various geological environments and can reach hazardous concentrations in groundwater and soils due to geochemical processes [1]. Globally, there are many endemic areas in low- and middle-income countries in Africa, Asia and Latin America with high exposure to fluoride from groundwater, with approximately 180 million people potentially affected worldwide [2]. In these countries with fluoride contamination problems in water, the following have been reported: damage to renal physiology [3, 4], decreased cognitive function in children of women exposed to fluoride during pregnancy [5], an increased risk of premature birth and low birth weight [6], and an increased risk of abortion in areas with high concentrations of fluoride [7]. Likewise, alterations in oxidative stress markers [8, 9] and decreased fertility in rats [10, 11] have been reported; however, information on placental alterations caused by fluoride exposure is very limited. However, there is available evidence indicating that fluoride in the mother’s blood crosses the placenta and is absorbed and excreted by the foetus [12]. The placenta is the temporary organ that fulfils the functions of hormone secretion, metabolic transfer, gas exchange and foetal protection and, therefore, is one of the organs most exposed to contaminant damage [13].
Nutrient transfer depends on the physical properties of the placenta [14]. Some studies suggest that fluoride exposure may indirectly affect placental function through effects on the reproductive and endocrine systems in female rats [11], while oxidative stress [15] and placental abnormalities, such as inflammation, hypercoiling and chronic villitis, may also contribute to placental insufficiency, having significant effects on pregnancy and foetal development [16].
Knowledge of how fluoride exposure affects placental development is limited; therefore, we hypothesized that fluoride affects placental structure and morphology, which impacts neonatal weight. A dysfunctional placenta can have a negative impact on neonatal weight, and it is important to monitor placental health during pregnancy to prevent adverse outcomes [17]. The objective of this work was to evaluate potential placental alterations caused by fluoride exposure in a rat model that simulates preconception and pregnancy exposure conditions in endemic areas.
Materials and Methods
Experimental Design
Fifteen female and eight male Wistar rats (250 ± 31.66 g) were obtained and maintained in the Laboratory Animal Experimentation and Production Unit (UPEAL-CINVESTAV) in accordance with NOM-062-ZOO-1999. The procedures were approved by the Internal Committee for the Care and Use of Laboratory Animals (CICUAL) of CINVESTAV; protocol number 0292-19. The animals were maintained in rooms at a constant temperature (21 ± 2 °C) humidity of 40–60%, with filtered air at 95% efficiency and noise level lower than 85 dB. Rats were provided with free access to rodent food (PicoLab® Mouse Diet 20, #5058, LabDiet®; Haward, CA) and purified water containing 10 μg fluoride per litre. Male rats without any treatment were used for mating.
Grouping and Fluoride Exposure
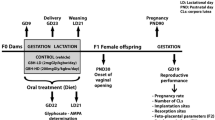
After 8 days of acclimatization, female rats were randomly assigned to three experimental groups (n = 5) as follows: control (received 2 ml/kg body mass of purified water by gavage); two exposed groups to sodium fluoride (Sigma–Aldrich, 99% pure) at doses equivalent to 2.5 and 5 mg fluoride/kg/d by gavage for 14 days. At day 14, the females and no exposed males were placed in the same cage at a ratio of 1:2; Pap smear was evaluated and the female rats with sperm observed in vaginal smears under a microscope were regarded as pregnant at day 1 gestation. The control and fluoride exposure group rats continued their treatment for 19 days after mating. Body weight, water intake and food consumption were monitored every 2 days. On gestation day 19 (GD19), under anaesthesia with ketamine/xylazine (80–5 mg/kg) intramuscularly, dystocic delivery was performed, and the gravid oviducts were extracted, from which the foetus and placenta were obtained (Fig. 1). The number, weight and height were recorded. The pregnant rats were euthanized postpartum. Placentas were stored at −70 °C until used for analysis.
Experimental design of rat exposure to fluoride. Wistar rats were exposed to fluoride by gastric gavage for 33 days. The administered dose was purified water, 2.5 and 5 mg fluoride/kg/d by gavage. The Pap smear was evaluated daily before mating. The presence of sperm in the Pap smear represented gestational day 1 (GD1); GD19, the delivery was performed.
Quantification of Fluoride in the Placenta
The placenta was homogenized by an Omni TH tissue homogenizer (PerkinElmer©) with Hard Tissue Omni Tip Plastic Homogenizing Probes (7×110 mm) (PerkinElmer©) at 35,000 rpm (500 mg in 1 mL of deionized water). The fluoride level in the placenta was evaluated in 25 placentas of each group after overnight hexamethyldisiloxane (HMDS)-facilitated diffusion [18], using a selective ion electrode (OrionTM Fluoride 9409BNWP, Thermo Fisher Scientific Inc.) coupled to a potentiometer. Standard reference material (PC-U-F2102) from the National Institute Public Health of Quebec (0.45 μg fluoride/mL) was prepared in duplicate and diffused in a manner like the samples. The samples and standard graph were prepared in equal proportions with TISAB II (total ionic strength adjustment buffer) solution. The mV reading was converted to μg fluoride using a standard graph. The limit of detection of fluoride in the placenta was 0.010 μg/g, the coefficient of variation was 13.25% and the accuracy was 101.47%.
Assessment of Malondialdehyde Levels in Placenta
A portion of placenta (25 mg) was homogenised by an Omni TH tissue homogenizer (PerkinElmer©) with Hard Tissue Omni Tip Plastic Homogenizing Probes (7×110 mm) (PerkinElmer©) at 35,000 rpm, on Nonidet-P40 lysis buffer (150 mM NaCl, 1% Triton X-100, 50mM Tris-HCl, pH 8.0) and COMPLETE protease inhibitors; the samples were sonicated for 10 s, centrifuged at 14,000 rpm for 10 min, and the supernatant was used for determination. Both the supernatant of the homogenate and the malondialdehyde (MDA) standard graph were processed in triplicate as indicated in the Thiobarbituric Acid Reactive Substances (TBARS) Assay kit (Cayman, chemical Item No.10009055). As a positive control, 50 μL of 3% hydrogen peroxide (H2O2) was added to 25 mg of placenta. The absorbance was read at 535 nm. The MDA concentration was expressed as μM per gram protein and calculated from a standard graph.
Stereological Analysis
Three random rat placentas from each group were evaluated and fixed in formaldehyde in 10% PBS buffer. Thereafter, the placentas were cut to obtain two half parts along the midline through the umbilical cord, and sections at 5 μm were obtained in semiseries, using one in every 60 sections, to avoid repetitive analysis of the same histological area. Cuts were fixed and stained with haematoxylin & eosin (H&E). Stained sections were captured with 4×, 10× and 40× magnifications using the High Resolution Light Optical Microscope Keyence BZ-X800 (Keyence Corporation of America, IL, USA) to produce full panoramic views.
To determine the central placental thickness, a central line was drawn perpendicular to the point of insertion of the umbilical cord, which crosses the entire placental sample, and then two lines were drawn parallel to it, one on each side, with approximately 800 μm. In the same way, the same three lines are drawn for evaluation of the labyrinth zone (LZ). The thickness of the LZ, the basal decidua (BD), the basal zone (BZ), the area of the LZ and the area of glucogenic cells (GC) were also measured using ImageJ v1.54 software, (National Institutes of Health, USA). Finally, vascular density was expressed as the ratio between the total number of vessels counted and the area of the LZ (vessels/mm2).
Evaluation of Vascular Endothelial Growth Factor in the Placenta
Placental homogenate was used to determine the vascular endothelial growth factor (VEGF-A) level. VEGF-A concentration was measured using the rat VEGF-A ELISA kit (ThermoFisher, RAT83785) according to the manufacturer’s instructions. Briefly, samples were warmed to room temperature (RT) and gently agitated, 100 μL of sample/standard were added to the wells with diluents and incubated at RT for 1 h. The wells were then washed, and the plates were incubated at RT for 1 h. The plates were then washed 5 times with a wash buffer and incubated with VEGF conjugate for 1 h at RT. After the washes, the plates were incubated with the substrate solution for 30 min at RT; the reaction was terminated with a stop solution and the absorbance was measured at 450 nm. VEGF-A concentration was expressed as pg/mg of protein and calculated from a standard four-parameter algorithm plot.
Statistical Analysis
Data are expressed as the median and interquartile range (IQR) or mean values ± standard deviation (SD). A one-way ANOVA or nonparametric test (Kruskal–Wallis test) was performed to determine the significance between groups. Significant value p ≤ 0.05; Tukey`s post hoc test was used to determine which groups were significantly different. All statistical analyses were performed using GraphPad Prism software version 8.0.2 (Boston, MA).
Results
Dam Gestational Parameters and Fluoride Exposure
After exposure, no significant differences in dam body weight were observed between the control and fluoride exposure groups (Table 1). The area under the curve (AUC) was calculated to assess the body weight gain of each rat during the time of exposure, and no differences were observed. In summary, our results show that fluoride exposure did not significantly affect the dam gestational parameters evaluated.
To examine the levels of fluoride that reached placental tissue, quantification of fluoride was conducted. The results showed a correspondence between fluoride exposure dose and fluoride concentration in placental tissue, finding a statistically significant increase in the mean fluoride quantity in the 5 mg fluoride/kg/d exposure group compared with the 2.5 mg fluoride/kg/d exposure group (Table 1). No detectable concentrations of fluoride were observed in the control group (<0.01 μg fluoride/g).
Foetus Parameters and Exposure Effects
Representative foetuses of the control and exposure groups are shown in Fig. 2. Apparent local erythema was observed in foetuses from dams exposed to 5 mg fluoride/kg/d (Fig. 2C) compared with the control and 2.5 mg fluoride/kg/d groups (Fig. 2A, B), in which foetuses showed a uniform pale pink colouration. A statistically significant decrease in the median weight (p < 0.0001) and height (p < 0.0001) of foetuses was observed in the 2.5 mg fluoride/kg/d exposure group compared with the control (Table 1), while foetuses in the 5 mg fluoride/kg/d exposure group showed a significant increase in weight (p < 0.001) and height (p < 0.0001).
Foetal-Placental Weight Relationship
To investigate placental efficiency, we analysed the relationship between foetal and placental weight in each group. As shown in Fig. 3, a positive and statistically significant correlation between placental and foetal weight was observed in the control group (Fig. 3A) and 5 mg fluoride/kg/d exposure group (Fig. 3C), while no significant correlation was observed in the 2.5 mg fluoride/kg/d exposure group (Fig. 3B). In addition, the foetal/placental weight ratio was significantly higher (p < 0.05) in the 5 mg fluoride/kg/d exposure group than in the control group and was lower in the 2.5 mg fluoride/kg/d exposure group (p < 0.0001) (Table 1).
MDA Levels in Placenta
To explore whether oxidative damage was produced in the placenta by fluoride exposure, levels of MDA, a recognized measure of lipid peroxidation, were analysed. Non-monotonic results were obtained in the concentration of MDA in the placental (Fig. 4). In the 2.5 mg fluoride/kg/d group, a statistically significant increase (p < 0.0001) in the concentration of MDA was observed compared with the control group. In contrast, exposure to 5 mg fluoride/kg/d did not significantly affect (p = 0.0689) the MDA concentration in the placenta.
Malondialdehyde (MDA) concentration in placenta. Bars represent mean ± SD of MDA concentration (μM) per gram of protein; hydrogen peroxide (3% H2O2) was used as positive control for MDA test; n = 15 placentas per group, experiments were performed in triplicate; *p < 0.01 significantly different by control group (one-way ANOVA test with post hoc Tukey test)
Placental Morphological Effects
To further investigate whether fluoride exposure is associated with placental alterations, morphological characteristics were evaluated, and the results are shown in Table 2. H&E-stained sections showed an increase (p < 0.05) in the width of the placenta from rats exposed to 5 mg fluoride/kg/d compared to the control group (3.5 vs. 3.2 mm), without significant changes in placental length. In the histological sections, the thickness of the BD and the LZ increased in the fluoride exposed groups, being significant [(p < 0.0001) and p < 0.001, respectively] in the 5 mg fluoride/kg/d group, while the BZ was significantly increased (p < 0.01) in the 2.5 mg fluoride/kg/d group. Furthermore, in the fluoride-treated groups, no differences were found in both the area of the glycogenic cell clusters and the area of the LZ (Table 2).
In addition, Fig. 5 shows representative micrographs of the three experimental groups, a panoramic image of the tested blood vessels and a close-up of the same sections to differentiate the placental zones (delimited by dotted lines) and some cell lines characteristic of the placental zone e.g. the LZ contains syncytiotrophoblast and cytotrophoblast, and the BZ is characterised by the presence of clusters of GC and trophoblast giant cells (TGC).
Histologic micrographs of rat placenta on gestational day 19. The dotted line delimits a placental zone. a Representative staining of placental slices at 4X from the control group; b representative staining of placental slices at 10× from the control group; c representative staining of placental slices at 4× from the 2.5 mg fluoride/kg/d; d representative placenta slice staining at 10× of the 2.5 mg fluoride/kg/d group; e representative placenta slice staining at 4× of the 5 mg fluoride/kg/d group; f representative placenta slice staining at 10× of the 5 mg fluoride/kg/d group; H&E stain, BV, blood vessel; BD, basal decidua; GC, glycogenic cells; TGC, trophoblastic giant cells; BZ, basal zone; LZ, labyrinth zone
Effects on the Placental Vasculature
To investigate whether vasculature of placenta was affected by fluoride exposure, the vascular density of the labyrinth area (VDZLa) was assessed. Our results show a marked decrease (p < 0.0001) in vascular density in the fluoride treated groups compared to the control. A significant difference was also observed between the 2.5 and 5 mg fluoride/kg/day groups (p < 0.0001) (Fig. 6).
Impact of maternal fluoride exposure on vascular density in the labyrinthine zone area. Bars represent mean ± SD of vascular density expressed in vessels/mm2. *p < 0.0001 significantly different from the control group (one-way ANOVA test with post hoc Tukey test). #p < 0.0001 significantly different between fluoride exposure groups (one-way ANOVA test)
Finally, we examined the VEGF-A concentration in the placental tissue of the fluoride exposure groups. VEGF-A is an important factor involved in signalling pathways for vascular maintenance in placenta. Our finding revealed a statistically significant increase in VEGF-A concentration among the groups exposed to fluoride compared to the control group (Fig. 7). This increase was greater in the 2.5 mg fluoride/kg/d group (p < 0.05) than in the 5 mg fluoride/kg/d group (p < 0.05).
Discussion
The intrauterine effects of fluoride exposure are not well understood. In our study, we evaluated the potential placental alterations of fluoride exposure on a rat model that simulated exposure conditions prior to conception and during pregnancy in endemic areas. Our main finding is that fluoride exposure produced significant alterations in regard to foetal-placental weight ratio and placental morphology, as well as variations in vascular density and placental concentrations of VEGF-A. These effects exhibited distinctive differences depending on the dose of fluoride exposure, suggesting the possible activation of a different associated mechanism.
This study evaluated placental alterations on a rat model of biologically relevant oral fluoride concentrations. The concentrations of fluoride used were lower than the reported no-observed-adverse-effect level (NOAEL) for reproductive and developmental effects in Wistar rats, which is 8.5 to 13.7 mg fluoride/kg/d [19]. The concentrations used in the present study consist of a fluoride exposure of 21 and 42 ppm in water. In rodents, fluoride is distributed and cleared 5–10 times faster than in humans, and the effects are less toxic [20, 21]. Thus, the concentrations used in this study would be equal to human exposures at concentrations of 2.1 and 4.2 ppm, which can be found naturally in water contaminated with fluoride [22].
Placental efficiency, quantified by the ratio of foetal or birth weight to placental weight, is often used as a measure of placental efficiency in humans and animals and can be used as a proxy for how the placenta has adapted to meet foetal nutritional requirements [23, 24]. It is well recognized that both low or high changes in placental efficiency were associated with negative foetal development outcomes such as intrauterine growth restriction (IUGR), foetal death and low Apgar scores [23, 25]. In our study, opposite results regarding the weight and height of foetuses were noticed between the two doses of fluoride exposure compared with the control group. The decreased ratio of foetal/placental weight and the noncorrelation of them in the group exposed to 2.5 mg fluoride/kg/d could indicate a failure of placental efficiency, which implies that there may be a decrease in placental nutrient transfer. On the other hand, while this relationship was reestablished within the 5 mg fluoride/kg/d group, the increased foetal/placental weight ratio and the presence of erythema in the exposed foetuses lead us to hypothesise that the apparent increase in “efficiency” reflects a possible occurrence of inflammatory process in the foetus, rather than a genuine biological efficiency [23]. On the other hand, previous experimental studies that investigated the effects of intrauterine fluoride exposure, yielded inconsistent results on foetal outcomes such as foetal weight and height [6, 26]. These variations in outcomes, when compared to our findings, could potentially be attributed to differences in the dose of fluoride exposure employed in these studies. Furthermore, it is worth noting that none of these previous studies have specifically investigated alterations of fluoride at the placental level directly. Nonetheless, it is evident that further research is needed to provide a more compressive effect of fluoride on placental function.
To better understand the placental changes associated with fluoride exposure, we conducted an analysis focusing on some structural characteristics. In our study, we observed an increase in placental weight and changes in the thickness of the placental areas. The placental thickness is a nonspecific finding, however, variations in both reductions and increases have been associated with negative pregnancy outcomes caused by exposure to several toxicants that disrupt placental structure on multiple levels [27,28,29,30,31]. Our findings showed that exposure to 5 mg fluoride/kg/d increases the thickness of BD and LZ, while the exposure to 2.5 mg fluoride/kg/d only increases the thickness of BZ. Experimental studies have shown that under conditions of sustained damage to the placenta, the BD zone spongiotrophoblast proliferates and migrates towards both the BZ and the LZ, resulting in the thickening of the placental zones [32]. This migration serves as a compensatory mechanism to offset the reduction in nutrient exchange and maternal blood flow to attend foetal demands and potentially mitigate the effects of impaired foetal growth [27, 32, 33]. It is noteworthy that the observed increase of thickness in placental zones is mainly attributed to hormonal imbalances, given that these zones are regulated by hormones [34]. Furthermore, it seems probably that the ultrastructural changes in our study are connected to the distinct relationship between foetal and placental weights observed in response to each dosage of fluoride exposure. This, in turn, may be attributed to the activation of varying mechanisms of fluoride toxicity and placental adaptation processes. Nonetheless, further research is necessary to elucidate this relationship. In addition, the fluoride capacity to induce placental adaptation mechanisms could be explained through the generation of oxidative stress or through the reduction of oestrogen levels [35, 36]. These mechanisms have the potential to generate structural and morphological changes in the placenta, which may serve as part of a compensatory mechanism [37].
Optimal placental vascularization has been related to the establishment of a successful pregnancy and its alteration has been directly associated with changes in placental function and the activation of adaptive mechanisms. Our findings showed a decrease in the VDLZa and an increase in placental concentration of VEGF-A in the fluoride exposure groups, both parameters associated with placental vasculature. It is important to note that regions exhibiting decreased vascular density correspond to regions characterised by compromised maternal perfusion and inadequate oxygenation in utero [38]. Importantly, these variations in vascular density can be associated with alterations in molecules that mediate placental vascular maintenance, including VEGF-A. This factor plays an important role in vasculogenesis and angiogenesis, and its imbalance has been linked with hypoxia and adverse outcomes such as preeclampsia [39, 40]. In this context, our results suggest that fluoride exposure could affect placental vasculature, potentially resulting in an impact on placental efficiency and function.
Oxidative stress is continuously generated in the placenta, but its overproduction can damage biomolecules, accelerating placental insufficiency and therefore compromising foetal viability [15]. MDA is a product of lipid peroxidation and has been widely used as an oxidative damage marker. The increase of MDA levels associated with oxidative stress generation after fluoride exposure has been reported in several studies [8]. However, in our study, we only observed a significant increase in placenta concentration of MDA in the group exposed to 2.5 mg fluoride/kg/d. We hypothesised that different results in the 5 mg fluoride/kg/d group might be caused by the activation of different toxicity mechanisms or the activation of adaptive mechanisms. The decrease of oxidative damage characterised by a decrease in the levels of MDA in placenta tissue has previously been reported in obese and diabetic pregnancies; these studies have suggested the activation of protective or adaptive mechanisms that prevent oxidative damage produced, such as nitrosative stress [41, 42]. On the other hand, Chouhan and Flora [43] have reported that oxidative stress mediates fluoride toxicity at low levels of fluoride exposure in soft tissues but not in higher doses. This suggested that different modes of action depend on the fluoride exposure dose. This could also explain the absence of a dose–response relationship observed in placental MDA concentration with increased fluoride exposure.
In summary, our findings showed a differential response of the evaluated placenta parameters to fluoride exposure. This suggests that fluoride may affect foetal development through a biphasic dose–response, where adaptive mechanisms and different mechanisms of fluoride-induced toxicity, are both activated. A research conducted by Ortiz-García and colleagues has reported a nonlinear association of maternal urinary fluoride concentration with birth weight [44]. The study demonstrated that the association of fluoride exposure with changes in birth weight was dependent on the trimester of pregnancy and the breakpoints of the fluoride exposure levels. These findings also suggest a possible dual effect of exposure to different levels of fluoride during pregnancy. The biphasic effects of fluoride have been recognized in other organs and could be linked with the activation of different mechanisms of toxicity [10, 43, 45]. However, as we discussed previously, we do not discard the potential activation of adaptive mechanisms in response to fluoride-induced damage [24].
Alternatively, it has been reported that fluoride can induce systemic vascular alterations such as atherosclerosis and blood pressure modification [46, 47]. It is plausible that those systemic effects may also be present during pregnancy and potentially contribute to alterations in the placenta and umbilical cord, leading to modifications of foetal blood flow [48, 49]; however, this has not yet been studied. Recently, the effect of fluoride exposure on human intrauterine development has gained importance. Clearly, our understanding of the mechanisms associated with placental alterations of fluoride exposure, as well as the potential effects on development and growth outcomes after birth remains incomplete and requires further investigation. Nevertheless, this study reinforces the findings that exposure to fluoride during pregnancy could affect foetuses [6, 44] and offers important insights that can be helpful to guide further research.
To our knowledge, this is the first study to propose a model of fluoride exposure conditions during pregnancy to identify placental and foetal alterations. Nevertheless, we acknowledge that our study has limitations. First, at the vascular level, we limited ourselves to counting the number and determining the VDLZa; however, we did not evaluate the vascular damage. Second, at a methodological level, we limited ourselves to determining placental weight and length, while there are other parameters, such as Doppler velocimetry, of the impedance of blood flow in the umbilical artery that we could have used to determine whether these changes in placental and foetal weights were due to poor placental perfusion [50, 51]. Last, placenta structures in rats may be more resistant to placental disruption than placental structures in humans due to rats’ resistance to fluoride exposure [52]. Thus, whether fluoride can cause alterations in placentation, defects in the contractility of the placental vasculature, apoptosis of invading cytotrophoblasts, or inadequate remodelling of spiral arteries, alterations that are well known to lead to decreased placental blood flow [53], which is associated with the development of pathophysiological processes, remains to be studied.
Conclusions
In conclusion, the results of this study showed that exposure to environmentally relevant concentrations of fluoride produces significant non-monotonic changes in the foetal-placental weight ratio, affects the thickness of the placental area, increases VEGF-A levels, and causes a decrease in the VDLZa. In addition, we observed an inadequate foetal growth in both doses and only an increase of MDA in the placenta at lower exposure dose. These findings are indicative of alterations in both placental morphology and efficiency, modifications that are related to later developmental defects. This study deepens the knowledge of the effects of fluoride toxicity on the placenta and its impact on foetuses and provides new avenues for the study of the effects of toxicity during gestation.
References
Limon-Pacheco JH, Jimenez-Cordova MI, Cardenas-Gonzalez M, Sanchez Retana IM, Gonsebatt ME, Del Razo LM (2018) Potential co-exposure to arsenic and fluoride and biomonitoring equivalents for Mexican children. Ann Glob Health 84:257–273. https://doi.org/10.29024/aogh.913
Podgorski J, Berg M (2022) Global analysis and prediction of fluoride in groundwater. Nat Commun 13:4232–4241. https://doi.org/10.1038/s41467-022-31940-x
Cardenas-Gonzalez M, Jacobo Estrada T, Rodriguez-Munoz R, Barrera-Chimal J, Bobadilla NA, Barbier OC, Del Razo LM (2016) Sub-chronic exposure to fluoride impacts the response to a subsequent nephrotoxic treatment with gentamicin. J Appl Toxicol 36:309–319. https://doi.org/10.1002/jat.3186
Jimenez-Cordova MI, Cardenas-Gonzalez M, Aguilar-Madrid G, Sanchez-Pena LC, Barrera-Hernandez A, Dominguez-Guerrero IA, Gonzalez-Horta C, Barbier OC, Del Razo LM (2018) Evaluation of kidney injury biomarkers in an adult Mexican population environmentally exposed to fluoride and low arsenic levels. Toxicol Appl Pharmacol 352:97–106. https://doi.org/10.1016/j.taap.2018.05.027
Valdez Jimenez L, Lopez Guzman OD, Cervantes Flores M, Costilla-Salazar R, Calderon Hernandez J, Alcaraz Contreras Y, Rocha-Amador DO (2017) In utero exposure to fluoride and cognitive development delay in infants. Neurotoxicol 59:65–70. https://doi.org/10.1016/j.neuro.2016.12.011
Sastry GM, Mohanty S, Varma Bhongir A, Mishra AK, Rao P (2011) Association of higher maternal serum fluoride with adverse fetal outcomes. Int J Med Public Health 1:13–17. https://doi.org/10.5530/ijmedph.2.2011.4
Sastry G, Shruti Mohanty M, Rao P (2010) Role of placenta to combat fluorosis (in fetus) In Endemic Fluorosis Area. Natl J Integr Res Med 1:16–19
Zhong N, Yao Y, Ma Y, Meng X, Sowanou A, Pei J (2020) Effects of fluoride on oxidative stress markers of lipid, gene, and protein in rats. Biol Trace Elem Res 199:2238–2246. https://doi.org/10.1007/s12011-020-02336-z
Sharma P, Verma PK, Sood S, Singh R, Gupta A, Rastogi A (2021) distribution of fluoride in plasma, Brain, and bones and associated oxidative damage after induced chronic fluorosis in Wistar rats. Biol Trace Elem Res 200:1710–1721. https://doi.org/10.1007/s12011-021-02782-3
Zhou Y, Qiu Y, He J, Chen X, Ding Y, Wang Y, Liu X (2013) The toxicity mechanism of sodium fluoride on fertility in female rats. Food Chem Toxicol 62:566–572. https://doi.org/10.1016/j.fct.2013.09.023
Zhou Y, Zhang H, He J, Chen X, Ding Y, Wang Y, Liu X (2013) Effects of sodium fluoride on reproductive function in female rats. Food Chem Toxicol 56:297–303. https://doi.org/10.1016/j.fct.2013.02.026
Castiblanco-Rubio GA, Martinez-Mier EA (2022) Fluoride Metabolism in pregnant women: a narrative review of the literature. Metabolites 12:1–13. https://doi.org/10.3390/metabo12040324
Burton GJ, Jauniaux E (2018) Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol 218:S745–S761. https://doi.org/10.1016/j.ajog.2017.11.577
Ma D, Lu Y, Liang Y, Ruan T, Li J, Zhao C, Wang Y, Jiang G (2022) A critical review on transplacental transfer of per- and polyfluoroalkyl substances: prenatal exposure levels, characteristics, and mechanisms. Environ Sci Technol 56:6014–6026. https://doi.org/10.1021/acs.est.1c01057
Sultana Z, Maiti K, Aitken J, Morris J, Dedman L, Smith R (2017) Oxidative stress, placental ageing-related pathologies and adverse pregnancy outcomes. Am J Reprod Immunol 77:1–10. https://doi.org/10.1111/aji.12653
Nikkels PG, Evers AC, Schuit E, Brouwers HA, Bruinse HW, Bont L, Houben ML, Kwee A (2021) Placenta pathology from term born neonates with normal or adverse outcome. Pediatr Dev Pathol 24:121–130. https://doi.org/10.1177/1093526620980608
Maltepe E, Fisher SJ (2015) Placenta: the forgotten organ. Annu Rev Cell Dev Biol 31:523–552. https://doi.org/10.1146/annurev-cellbio-100814-125620
Taves DR (1968) Separation of fluoride by rapid diffusion using hexamethyldisiloxane. Talanta 15:969–974. https://doi.org/10.1016/0039-9140(68)80097-9
Guth S, Huser S, Roth A, Degen G, Diel P, Edlund K, Eisenbrand G, Engel KH, Epe B, Grune T et al (2020) Toxicity of fluoride: critical evaluation of evidence for human developmental neurotoxicity in epidemiological studies, animal experiments and in vitro analyses. Arch Toxicol 94:1375–1415. https://doi.org/10.1007/s00204-020-02725-2
Dionizio AS, Melo CGS, Sabino-Arias IT, Ventura TMS, Leite AL, Souza SRG, Santos EX, Heubel AD, Souza JG, Perles J et al (2018) Chronic treatment with fluoride affects the jejunum: insights from proteomics and enteric innervation analysis. Sci Rep 8:3180–3192. https://doi.org/10.1038/s41598-018-21533-4
Appendix D (2006) Comparative Pharmacokinetics of Rats and Humans. In Fluoride in Drinking Water: A Scientific Review of EPA's Standards. Nat Res Council:442–446
Gonzalez-Horta C, Ballinas-Casarrubias L, Sanchez-Ramirez B, Ishida MC, Barrera-Hernandez A, Gutierrez-Torres D, Zacarias OL, Saunders RJ, Drobna Z, Mendez MA et al (2015) A concurrent exposure to arsenic and fluoride from drinking water in Chihuahua, Mexico. Int J Environ Res Public Health 12:4587–4601. https://doi.org/10.3390/ijerph120504587
Haavaldsen C, Samuelsen SO, Eskild A (2013) Fetal death and placental weight/birthweight ratio: a population study. Acta Obstet Gynecol Scand 92:583–590. https://doi.org/10.1111/aogs.12105
Hayward CE, Lean S, Sibley CP, Jones RL, Wareing M, Greenwood SL, Dilworth MR (2016) Placental Adaptation: What can we learn from birthweight:placental weight ratio? Front Physiol 7:1–13. https://doi.org/10.3389/fphys.2016.00028
Shehata F, Levin I, Shrim A, Ata B, Weisz B, Gamzu R, Almog B (2011) Placenta/birthweight ratio and perinatal outcome: a retrospective cohort analysis. BJOG 118:741–747. https://doi.org/10.1111/j.1471-0528.2011.02892.x
Helal M, Dakdoky ME (2006) Fetotoxicity of fluoride in rats and the protective action of some antioxidants. Fluoride 39:202–210
Furukawa S, Hayashi S, Usuda K, Abe M, Ogawa I (2008) Histopathological effect of ketoconazole on rat placenta. J Vet Med Sci 70:1179–1184. https://doi.org/10.1292/jvms.70.1179
Furukawa S, Hayashi S, Usuda K, Abe M, Ogawa I (2011) The relationship between fetal growth restriction and small placenta in 6-mercaptopurine exposed rat. Exp Toxicol Pathol 63:89–95. https://doi.org/10.1016/j.etp.2009.10.001
Sun J, Sugiyama A, Inoue S, Takeuchi T, Takeuchi T, Furukawa S (2013) Effect of methotrexate on rat placenta development. Exp Toxicol Pathol 65:995–1002. https://doi.org/10.1016/j.etp.2013.02.002
Monfared AL (2014) Histomorphological and ultrastructural changes of the placenta in mice exposed to formaldehyde. Toxicol Ind Health 30:174–181. https://doi.org/10.1177/0748233712452603
Shalaby AM, Ibrahim M, Aboregela AM (2019) Effect of aspartame on the placenta of adult albino rat. A histological and immunohistochemical study. Ann Anat 224:133–141. https://doi.org/10.1016/j.aanat.2019.04.007
Rosario GX, Konno T, Soares MJ (2008) Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol 314:362–375. https://doi.org/10.1016/j.ydbio.2007.12.007
Furukawa S, Hayashi S, Usuda K, Abe M, Hagio S, Ogawa I (2011) Toxicological pathology in the rat placenta. J Toxicol Pathol 24:95–111. https://doi.org/10.1293/tox.24.95
Furukawa S, Tsuji N, Sugiyama A (2019) Morphology and physiology of rat placenta for toxicological evaluation. J Toxicol Pathol 32:1–17. https://doi.org/10.1293/tox.2018-0042
Szczepanski M, Kamianowska M, Kamianowski G (2012) Effects of fluorides on apoptosis and activation of human umbilical vein endothelial cells. Oral Dis 18:280–284. https://doi.org/10.1111/j.1601-0825.2011.01873.x
Skorka-Majewicz M, Goschorska M, Zwierello W, Baranowska-Bosiacka I, Styburski D, Kapczuk P, Gutowska I (2020) Effect of fluoride on endocrine tissues and their secretory functions -- review. Chemosphere 260:127565–127577. https://doi.org/10.1016/j.chemosphere.2020.127565
Bartholomeusz RK, Bruce NW, Lynch AM (1999) Embryo survival, and fetal and placental growth following elevation of maternal estradiol blood concentrations in the rat. Biol Reprod 61:46–50. https://doi.org/10.1095/biolreprod61.1.46
Aughwane R, Schaaf C, Hutchinson JC, Virasami A, Zuluaga MA, Sebire N, Arthurs OJ, Vercauteren T, Ourselin S, Melbourne A et al (2019) Micro-CT and histological investigation of the spatial pattern of feto-placental vascular density. Placenta 88:36–43. https://doi.org/10.1016/j.placenta.2019.09.014
Meng Q, Shao L, Luo X, Mu Y, Xu W, Gao L, Xu H, Cui Y (2016) Expressions of VEGF-A and VEGFR-2 in placentae from GDM pregnancies. Reprod Biol Endocrinol 14:61–70. https://doi.org/10.1186/s12958-016-0191-8
Melincovici CS, Boşca AB, Şuşman S, Mărginean M, Mihu C, Istrate M, Moldovan IM, Roman AL, Mihu CH (2018) Vascular endothelial growth factor (VEGF) – key factor in normal and pathological angiogenesis. Rom J Morphol Embryol 59:455–467
Ramirez-Emiliano J, Fajardo-Araujo ME, Zuniga-Trujillo I, Perez-Vazquez V, Sandoval-Salazar C, Ornelas-Vazquez JK (2017) Mitochondrial content, oxidative, and nitrosative stress in human full-term placentas with gestational diabetes mellitus. Reprod Biol Endocrinol 15:26–34. https://doi.org/10.1186/s12958-017-0244-7
Santos-Rosendo, C.; Bugatto, F.; Gonzalez-Dominguez, A.; Lechuga-Sancho, A.M.; M, M.R.; Visiedo, F. Placental adaptive changes to protect function and decrease oxidative damage in metabolically healthy maternal obesity. Antioxidants (Basel) 2020, 9, 794-811, https://doi.org/10.3390/antiox9090794.
Chouhan S, Flora SJ (2008) Effects of fluoride on the tissue oxidative stress and apoptosis in rats: biochemical assays supported by IR spectroscopy data. Toxicology 254:61–67. https://doi.org/10.1016/j.tox.2008.09.008
Ortiz-Garcia SG, Torres-Sanchez LE, Munoz-Rocha TV, Mercado-Garcia A, Peterson KE, Hu H, Osorio-Yanez C, Tellez-Rojo MM (2022) Maternal urinary fluoride during pregnancy and birth weight and length: results from ELEMENT cohort study. Sci Total Environ 838:156459–115653. https://doi.org/10.1016/j.scitotenv.2022.156459
Al-Hiyasat AS, Elbetieha AM, Darmani H (2000) Reproductive toxic effects of ingestion of sodium fluoride in female rats. Fluoride 33:79–84
Liu H, Gao Y, Sun L, Li M, Li B, Sun D (2014) Assessment of relationship on excess fluoride intake from drinking water and carotid atherosclerosis development in adults in fluoride endemic areas China. Int J Hyg Environ Health 217:413–420. https://doi.org/10.1016/j.ijheh.2013.08.001
Yousefi M, Yaseri M, Nabizadeh R, Hooshmand E, Jalilzadeh M, Mahvi AH, Mohammadi AA (2018) Association of hypertension, body mass index, and waist circumference with fluoride intake; water drinking in residents of fluoride endemic areas Iran. Biol Trace Elem Res 185:282–288. https://doi.org/10.1007/s12011-018-1269-2
Koech A, Ndungu B, Gichangi P (2008) Structural changes in umbilical vessels in pregnancy induced hypertension. Placenta 29:210–214. https://doi.org/10.1016/j.placenta.2007.10.007
Salih MM, Ali LE, Eed EM, Siniyeh AA (2022) Histomorphometric study of placental blood vessels of chorion and chorionic villi vascular area among women with preeclampsia. Placenta 124:44–47. https://doi.org/10.1016/j.placenta.2022.05.011
Chimini JS, Possomato-Vieira JS, da Silva MLS, Dias-Junior CA (2019) Placental nitric oxide formation and endothelium-dependent vasodilation underlie pravastatin effects against angiogenic imbalance, hypertension in pregnancy and intrauterine growth restriction. Basic Clin Pharmacol Toxicol 124:385–393. https://doi.org/10.1111/bcpt.13149
Salavati N, Smies M, Ganzevoort W, Charles AK, Erwich JJ, Plosch T, Gordijn SJ (2019) The possible role of placental morphometry in the detection of fetal growth restriction. Front Physiol 9:1884–1896. https://doi.org/10.3389/fphys.2018.01884
Perera T, Ranasinghe S, Alles N, Waduge R (2018) Effect of fluoride on major organs with the different time of exposure in rats. Environ Health Prev Med 23:1–9. https://doi.org/10.1186/s12199-018-0707-2
Vangrieken P, Remels AHV, Al-Nasiry S, Bast A, Janssen GMJ, von Rango U, Vroomans D, Pinckers YCW, van Schooten FJ, Schiffers PMH (2020) Placental hypoxia-induced alterations in vascular function, morphology, and endothelial barrier integrity. Hypertens Res 43:1361–1374. https://doi.org/10.1038/s41440-020-0528-8
Acknowledgements
We thank Elizabeth H. Choy (University of California Irvine, Irvine, CA, USA) for her invaluable support in the images taken under the microscope. We also acknowledge the assistance of Angel Barrera-Hernández, Maricela Uribe-Ramirez, and Esaú Montañez-Rodriguez. J.G.A. held a Conacyt PhD. Fellowship No. 727322.
Funding
This study received no external funding; it was funded by Centro de Investigación y Estudios Avanzados del Instituto Politécnico Nacional.
Author information
Authors and Affiliations
Contributions
J.G.A. and L.M.D.R. conceived and designed the study. Data collection and analysis were performed by J.G.A., O.G.A.A. and M.I.J.C.; J.G.A. and M.I.J.C. wrote the original draft. L.M.D.R. reviewed and edited the manuscript. All authors read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All experimental procedures were approved by the Institutional Animal Care and Use Committee (CICUAL, Protocol# 0292-19) in accordance with the Mexican Guideline Regulations of Animal Care and Maintenance (NOM-062-ZOO-1999) and the international guidelines for the use and care of laboratory animals as adopted and promulgated by the US National Institutes of Health.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guerrero-Arroyo, J., Jiménez-Córdova, M.I., Aztatzi-Aguilar, O.G. et al. Impact of Fluoride Exposure on Rat Placenta: Foetal/Placental Morphometric Alterations and Decreased Placental Vascular Density. Biol Trace Elem Res 202, 3237–3247 (2024). https://doi.org/10.1007/s12011-023-03916-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03916-5