Abstract
The current study evaluated the potential ameliorative effect of a dietary immune modulator, Nannochloropsis oculata microalga, on the mercuric chloride (HgCl2)-induced toxicity of Nile tilapia. Nile tilapia (45–50 g) were fed a control diet or exposed to ¼ LC50 of HgCl2 (0.3 mg/L) and fed on a medicated feed supplemented with N. oculata (5% and 10% (50 or 100 g/kg dry feed)) for 21 days. Growth and somatic indices, Hg2+ bioaccumulation in muscles, and serum acetylcholinesterase (AChE) activity were investigated. Antioxidant and stress-related gene expression analyses were carried out in gills and intestines. Histopathological examinations of gills and intestines were performed to monitor the traits associated with Hg2+ toxicity or refer to detoxification. Hg2+ toxicity led to significant musculature bioaccumulation, inhibited AChE activity, downregulated genes related to antioxidants and stress, and elicited histopathological changes in the gills and intestine. Supplementation with N. oculata at 10% was able to upregulate the anti-oxidative-related genes while downregulated the stress apoptotic genes in gills and intestines compared to the unexposed group. In addition, minor to no histopathological traits were detected in the gills and intestines of the N. oculata-supplemented diets. Our data showed the benefit of dietary N. oculata in suppressing Hg2+ toxicity, which might support its efficacy as therapeutic/preventive agent to overcome environmental heavy metal pollution in aquatic habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish environmental diseases are caused by numerous ecological contaminants, including heavy metals [1]. Aquatic contamination by heavy metals might happen from atmospheric deposition or industrial activities; therefore, their bioaccumulation was frequently reported in different aquatic ecosystems worldwide [2,3,4]. Several anthropogenic activities, such as fossil fuel and coal combustions or agricultural and industrial consumptions, release heavy metals causing severe contamination of the adjacent aquatic habitats. These metals are dangerous for aquatic organisms as they could persist in their environment and accumulate in their organs, negatively affecting their health, growth, meat quality, and reproduction and leading to death or extended harm to their offspring [5,6,7]. Furthermore, the damage of such elements extends to the human consumers of fish and seafood [8,9,10] or through agricultural product consumption [11].
Mercury (Hg) is one of the toxic heavy metals that spread out in aquatic habitats mainly from agricultural waste containing pesticides, in addition to occurring naturally in the atmosphere and could be deposited via weathering [6]. It is considered the third most dangerous environmentally heavy metal pollutant after arsenic and lead, found in the environment in three different forms elementary (Hg0), organic (methylmercury “MeHg”), and inorganic (chloride mercury “HgCl2”). Although MeHg is the most toxic form [12], HgCl2 is the most common and harmful form since it can pass across biological membranes and interact with amino acids forming organo-mercury complexes [13]. The reported Hg2+ toxicity for fish is in the form of brain oxidative stress, inhibition of hepatic biotransformation enzymes, genotoxicity in blood, and reproductive alterations [14,15,16].
Several factors can cause Hg2+ accumulation in fish tissues. Environmental factors such as pH of the water, dissolved organic carbon, and other biological factors including fish age, size, foraging habitat, and primary productivity can all influence bioaccumulation [6, 17]. Low primary productivity leads to high Hg2+ concentrations because of the algal bio-dilution, which increases the accumulated MeHg levels [6]. Additionally, Hg2+ concentrations increased with fish size, and higher concentrations were reported in the bottom (demersal) than in the surface (pelagic) fish and concerning percent floodable area rather than the hydromorphic soils, which did not influence fish Hg level [6].
The inorganic mercury compounds suppress the antioxidant defense system in host bodies and induce apoptosis. Chelating agents are used in medicine to treat mercury toxicity by forming chelation compounds with toxic metal ions that are easily excreted through the excretory system [13]. On the other hand, natural antioxidants are preferable because drugs are ineffective at repairing tissue damage and may cause toxic side effects. Using the biological systems for mercury absorption reported augmentation of Hg2+ accumulation in the aquatic organisms’ organs [18].
Several natural antioxidant products have recently been used as potential non-toxic therapies against heavy metals and microalgae toxicity. Microalgae are essential metabolites for food and medicine [19] and have gained high importance in the aquaculture industry [20] since they were frequently used as feed and food additives, in addition to being live feed in aquaculture [21]. Furthermore, surfaces of algal cells possess several kinds of functional groups responsible for heavy metals’ chelation from contaminated water [22]. Microalgae, including N. oculata, rapidly respond to changes in element availability; therefore, they can be used to evaluate contaminated areas and predict the ecological effects of pollution.
The current study focuses on Hg2+ toxicity because it is a ubiquitous heavy metal; MeHg and HgCl2 naturally occur in the water and cause ultimate public health problems [23]. Besides, tilapia fish was selected as a model because it shows high resistance against water pollution during environmental toxicological studies [24, 25]. In line with our previous findings [26], the effects of mercury exposure have been studied extensively. Still, researchers have mostly focused on the cellular level, paying less attention to how exposure affects growth performances or gene expressions. Therefore, the primary goals of the present study were to examine the underlying molecular and genetic mechanisms and score analysis of the histopathological lesions. Further, the potential of the microalgae N. oculata as a natural protectant for HgCl2 pollutants was emphasized.
Materials and Methods
Diet Formulation
Ready-to-use N. oculata dry powder was purchased from the National Research Center, Cairo, Egypt. N. oculata-supplemented diets were prepared, as described in our previous study [26]. Two medicated diets were formulated by supplementing Nile tilapia basal feed with N. oculata powder at two different doses: 50 g/kg dry feed (i.e., 5% N. oculata) or 100 g/kg dry feed (i.e., 10% N. oculata). The dried pellets were placed in a plastic bag and stored at 4 °C until feeding. The composition of the medicated diets is presented in Table 1.
Fish Maintenance and Experimental Conditions
The current study was conducted at the experimental facility of the Aquatic Animal Medicine (AAM) Laboratory, Faculty of Veterinary Medicine, Mansura University, Mansoura, Egypt. Procedures for animal care and management were approved by the Ethical Research Committee of Mansoura University, Code number: MU-ACUC (VM.R.23.01.42).
Nile tilapia (O. niloticus, average total body weight 45–50 g, and length 14–15 cm) were obtained from a certified private fish farm at Kafr El Sheik Governorate. Fish were acclimated for two weeks to the de-chlorinated tap water in 500 L capacity fiberglass tanks supplied with adequate aeration (6.5–7.8 mg/L dissolved oxygen) and pH (7.1–7.3), and the water temperature was maintained at 24 ± 2 °C throughout the experiment. Fish were fed to satiation using commercial feed (Uccma feed, Egypt; crude protein, 32%; crude lipid, 6.2%; crude fiber, 5.7%) and were exposed to a 12 h light–dark cycle. During the acclimation period, fish were frequently checked, according to EJ Noga [27], and only healthy fish were chosen in terms of general appearance and activity level. Daily water parameters were monitored throughout the trial using water quality test kits (Aquarium Pharmaceuticals, Inc.) for each tank. Up to 50% of all aquaria’s water was siphoned and replaced twice weekly to maintain water quality parameters.
Fish Exposure to Mercury
The fish were exposed to a sub-lethal concentration of 0.3 mg/L HgC12 for up to 21 days. This concentration equates to 1/4th of the median lethal concentration of 1.21 mg/L based on a 96-h toxicological assay in Nile tilapia [26]. To summarize, a new daily stock solution of 1000 mg/L mercury was made by dissolving the calculated amount of HgCl2 in one liter of double-distilled water. The concentration in mg/L was then determined by adding a known volume of the stock solution to the glass aquaria. Fish not exposed to HgCl2 were kept in separate tanks under identical conditions.
Experimental Design
After acclimation, 120 selected healthy Nile tilapia fish were used for our experiment, which extended for 21 days. Twelve 80 L fiberglass indoor tanks with a primary stocking density of 10 fish per tank were randomly assigned into four groups in triplicate (i.e., 30 fish/group). Fish grouping conditions were kept considering similar “tank effects” between the replicate tanks. On the first day of the experiment, all groups were exposed for 21 days to the quarter value of the determined Hg2+ LC50 (i.e., 0.3 mg/L HgCl2), except one unexposed control group. Two groups received basal diets throughout the experiment, including the control group (G0) and one of the Hg2+ exposed groups (GHg). The other two groups were exposed to the same concentration of Hg2+ and simultaneously received medicated diets; a 5% N. oculata-supplemented diet was introduced to the third group (GN5+Hg), and a 10% N. oculata-supplemented diet was introduced to the fourth group (GN10+Hg). Throughout the experiment, fish were fed twice daily at 2% of their total body weight/day [28]. The leftover fish waste or feed was continuously siphoned from tanks, keeping constant clean water in all tanks. Up to 80% of the water in aquaria used to be replaced daily by a static-renewal system keeping the Hg2+ exposure state by adding a new daily stock solution of HgCl2 (0.3 mg/L) [29].
Fish Sampling and Tissue Collection
Two fish were sampled from each aquarium tank (6 fish/group) after 21 days of feeding. Sampled fish were euthanized with an overdose of MS222 (Argent) at 200 mg/L tricaine + 400 mg/L sodium bicarbonate. Each sampled fish was weighed and measured; then, the blood samples were withdrawn from their caudal vessels using a 23-gage needle. The collected blood was kept in an inclined position for 20 min at room temperature and then centrifuged at 1700 × g for 10 min for serum separation and stored at − 20 °C until analysis. Fish were dissected immediately after euthanasia, and the muscles, gills, and intestine were excised. The muscles were immediately processed for the estimation of Hg2+ bioaccumulation. The gills and intestines were cut into small portions. Some portions were placed in RNA Later® (Qiagen) and kept overnight at 4 °C and then stored at − 80 °C until further use for the gene expression analysis. Other portions were fixed in 10% neutral buffer formalin for the processing of the histopathological examination.
Fish Growth and Somatic Indices
Fish initial and final weight and length were estimated for each sampled fish to follow up on the fish growth performance. The condition factor (K-factor) was calculated according to the following formula: K-factor = [(fish weight) (g)/ (fish length (cm))3] × 100. In addition, the liver was excised immediately upon dissection and weighed to determine the HSI according to the following formula: HSI = (liver weight (g)/ body weight (g)) × 100.
Total Mercury Content in Nile Tilapia Musculature
Digested samples consisted of 1 g of fish musculature placed in 5 mL of 65% nitric acid (HNO3) in a water bath maintained at 100 °C until a clear liquid was obtained [30]. After cooling, the fluid was filtered through Whatman filter paper (No. 42, pore size 2.5 m). Following dilution in deionized water to a final volume of 25 mL, the Hg concentration was determined using an atomic absorption spectrophotometer (AAS- Perkin Elmer Analyst 100) fitted with an MHS-10 mercury/hydride system.
Serum Acetylcholinesterase (AChE) Activity
Acetylcholinesterase activity was estimated to evaluate organic poisoning as a biological indicator of intoxication [31]. The collected serum cholinesterase was measured following the manufacturer’s protocol of the Cobas c pack reagents using COBAS INTEGRA 400 plus analyzer Roche Diagnostics. Briefly, serum cholinesterase enzymatic activity was measured by continuous colorimetric kinetic methodology. It is based on a kinetic test by the method of butyrylthiocholine. The color decrease was measured in wavelength between 405 and 415 nm. The results were expressed in international units per liter (U/L).
Gene Expression Analysis
Two antioxidant enzymes-relevant genes, glutathione-S-reductase (GSR) and glutathione peroxidase (GPx) genes, and two stress proteins-relevant encoding genes, heat-shock protein (HSP) and CRISPR-encoded protein (caspase 3, CAS) genes, were targeted for our study. The RNA was extracted from intestinal and gill tissues using RNAlater (Invitrogen; Thermo Fisher Scientific, Inc.), and cDNA synthesis was performed using TOPscript™ RT DryMIX (dT18/dN6 plus). Quantitative real-time PCR (qPCR) was conducted according to the manufacturer’s instructions in real-time PCR system (The Azure Biosystems Cielo™ qPCR Systems, USA) using TOPreal™ qPCR 2X PreMIX (SYBR Green with low ROX). The PCR cycling conditions included a primary denaturation at 95 °C for 15 min followed by 40 series of denaturation at 95 °C for 30 s, annealing at 60 °C for 60 s, and extension at 72 °C for 60 s. The oligonucleotide-specific primers were previously published; GSR, GPx, HSP, β-actin [32, 33], and CAS [34]. A standard curve for each gene was established to quantify the expression levels. The expression levels of each gene were calculated as arbitrary units normalized to that of β-actin. Relative expressions were calculated by comparing the average expression level of the experimental group with that of the corresponding control group.
Histopathological Examination and Scoring System
The intestine and gills were dissected from the Nile tilapia and then collected and fixed in 10% neutral buffered formalin for 24 h. The dissected organs were placed in tissue cassettes, processed, and embedded in paraffin wax. A microtome (Leica RM2125 RTS, Germany) sliced embedded samples at 5 µm. The slices were stained using hematoxylin and eosin [35]. The stained slides were examined under a light microscope (Olympus CX 31 microscope). Additionally, the positively stained goblet cells were counted in intestinal sections of different experimental groups. The numbers were counted in four fields of view at 400 × magnifications and expressed as the mean goblet cell number per 6 fields of view. In this experiment, the lesions were analyzed in four fields of view at magnification 400 × for the gills and intestine section of three fish (3 sections per slide for each fish) in 12 fields of view per fish. Basic histopathological scoring parameters for lesions have been conducted as in Tables 2 and 3. The scale value of each lesion was determined for each fish (none: 0, mild: 1, moderate: 2, and severe: 3). The average of all scores was considered per fish [36].
Statistical Analysis
Data were first subjected to normality and homogeneity checks using Kolmogorov–Smirnov and Levene’s tests. All data, excluding the histopathological scoring, were subjected to a one-way analysis of variance (ANOVA) followed by a Tukey post hoc test to compare means between groups using GraphPad Prism v 8.4.2 (GraphPad Software, Inc., USA). Normalized individual fold change values were anchored to the lowest value recorded in each data set and then Log2 transformed, as described previously [37]. Nonparametric Kruskal–Wallis and Mann–Whitney U tests were used to analyze the histopathological scoring data among treatment groups and between two group comparisons. One-way ANOVA using post hoc Tukey’s multiple range tests was used to compare the mean values among experimental groups, including goblet cell numbers. All data were expressed as mean ± standard deviation (SD). Differences were considered statistically significant when p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), and p < 0.0001 (****).
Results
Fish Growth Performance
Dietary N. oculata for 21 days did not significantly affect the fish growth and somatic indices, where no statistical changes were observed in the final body weight between groups. In addition, no apparent influence was observed on the K-factor and HSI of the exposed fish compared to each other or compared to the control unexposed fish (Table 4).
Bioaccumulation in Fish Muscles
Hg2+ accumulated in the exposed, non-supplemented tilapia muscles up to 1.5 mg/kg of their body weight. Dietary N. oculata decreased the Hg2+ content to approximately a third of its exposed group level as N. oculata 5%, and 10% supplementing doses decreased the accumulated amount to 0.5 and 0.8 mg/kg, respectively. Notably, small traces (0.04 mg/kg) were recorded in the muscles of the control unexposed, untreated fish (Fig. 1).
The effects of HgCl2 exposure and diets on Hg2+ accumulation in Nile tilapia. The fish were fed with the control diet (G0) or exposed to 0.3 ppm HgCl2 and fed with the control diet (GHg), or with diets containing 5% N. oculata (GN5+Hg) or 10% N. oculata (GN10+Hg) for 21 days. Data are expressed as the mean ± SEM of six fish. Values with a different letter superscript are significantly different between groups. Significant levels (p < 0.05, 0.01, and 0.001), as determined by one-way ANOVA
Acetylcholinesterase (AChE) Activity
A significant decrease was recorded in the AChE activity of all the exposed fish compared to the control, regardless of N. oculata dietary supplementation. Notably, N. oculata supplementation was able to augment the AChE levels, and the GN10+Hg recorded higher augmentation than the GN5+Hg; however, it is of no significance (Fig. 2).
The effects of HgCl2 exposure and diets on serum acetylcholinesterase (AChE) in Nile tilapia. The fish were fed with the control diet (G0) or exposed to 0.3 ppm HgCl2 and fed with the control diet (GHg), or with diets containing 5% N. oculata (GN5+Hg) or 10% N. oculata (GN10+Hg) for 21 days. Data are expressed as the mean ± SEM of six fish. Values with a different letter superscript are significantly different between groups. Significant levels (p < 0.05, 0.01, and 0.001), as determined by one-way ANOVA
Relative Expression of the Antioxidant, Stress, and Apoptotic-Relevant Genes
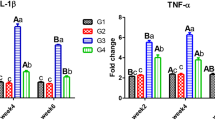
Gene expression analysis in gills and intestine tissues is presented in Fig. 3. In our findings, Hg2+ exposure significantly downregulated the antioxidant enzymes related genes in gills and intestine (GSR, p < 0.001), (GPx p = 0.001, p = 0.038, in gills and intestine, respectively), while upregulated the stress-related genes, CAS (p = 0.006, p = 0.037, in gills and intestine, respectively), and HSP (p < 0.001, p = 0.008, in gills and intestine, respectively) compared to the unexposed control group (G0).
The effects of HgCl2 exposure and diets on the expression of genes related to antioxidant (glutathione-S-reductase (GSR) and glutathione peroxidase (GPx)), stress (heat-shock protein (HSP 70)), and apoptosis (CRISPR-encoded protein (caspase 3, CAS)) in Nile tilapia. The fish were fed with the control diet (G0) or exposed to 0.3 ppm HgCl2 and fed with the control diet (GHg), or with diets containing 5% N. oculata (GN5 + Hg) or 10% N. oculata (GN10+Hg) for 21 days. Data are expressed as the mean ± SEM of six fish. Values with a different letter superscript are significantly different between groups. Significant levels (p < 0.05, 0.01, and 0.001), as determined by one-way ANOVA
Our results revealed that 10% dietary N. oculata revealed a significant elevation in the GSR gene expression of both gills and intestines compared to the other groups (GHg and GN5+Hg) (p < 0.001). Notably, this elevation was high in gills and recorded a value close to and not significantly different (p > 0.05) than that of the G0 group. Similarly, 5% and 10% dietary N. oculata elevated the expression of the GPx gene in both gills and intestines compared to GHg. This elevation was insignificant in the gills of GN5+Hg and GN10+Hg (p = 0.127 and p = 0.082, respectively). At the same time, it was significant in the intestine of GN5+Hg and GN10+Hg (p = 0.028 and p = 0.017, respectively), and both recorded values close to the G0 group without significant difference among them.
As for the immune-relevant genes, a significant decrease in the relative expression of the HSP-encoding gene in both gills and intestine of GN5+Hg (p < 0.001 and p < 0.05, respectively) and GN10+Hg (p < 0.001 and p = 0.031, respectively) compared to GHg group. No significant differences (p > 0.05) were recorded among the G0, GN5+Hg, and GN10+Hg groups regarding the relative expression of the HSP gene. On the other hand, 10% N. oculata-supplemented diets significantly downregulated the relative expression of the CAS-encoding gene in both the intestine and the gills compared with the GHg and GN5+Hg groups (p = 0.006 and p = 0.027 for gills and intestine, respectively). Notably, in the intestine, no significant differences (p = 0.53) were recorded between GN5+Hg and GN10+Hg and with the G0 group in the relative expression of the CAS gene (p = 0.659 and p = 0.995, respectively).
Histopathological and Scoring Results
Histopathological examination of the intestine and gills was investigated to confirm the protective effect of N. oculata against mercury toxicity. The histopathological score was performed for the intestine and gills (Fig. 4A–C). A significant difference in pathological effect on gills and intestine were observed between treatment groups with p = 0.001 (Figs. 4A and 5). The pathological effect of GHg on gills and intestines was significantly evident compared to the control group (p = 0.050 and p = 0.034, respectively). However, a significant reduction of the histopathological effect was detected in N. oculata groups compared to GHg with insignificant difference compared to the control group (p = 1.000, in the intestine and gills of GN5+Hg) and (p = 0.099, in the intestine, p = 0.453 in the gills of GN10+Hg). The gills of the control group are showing the normal histological appearance of primary and secondary lamellae (Fig. 5A). Diffuse lamellar fusion and lymphocytic bronchitis were detected in GHg group (Fig. 5B and C). However, few pathological findings were seen in GN5+Hg and GN10+Hg groups (Fig. 5D and E).
The effects of HgCl2 exposure and diets on pathological scores among the groups in gills (A), intestines (B), and goblet cells (C) in Nile tilapia. The fish were fed with the control diet (G0) or exposed to 0.3 ppm HgCl2 and fed with the control diet (GHg), or with diets containing 5% N. oculata (GN5 + Hg) or 10% N. oculata (GN10+Hg) for 21 days. Data are expressed as the mean ± SEM of three fish. Values with a different letter superscript are significantly different between groups. Significant levels (p < 0.05, 0.01, and 0.001), as determined by Kruskal–Wallis and Mann–Whitney U tests
Histopathology of HgCl2 exposure in the gills of Nile tilapia. The fish were fed with the control diet (G0) or exposed to 0.3 ppm HgCl2 and fed with the control diet (GHg), or with diets containing 5% N. oculata (GN5 + Hg) or 10% N. oculata (GN10+Hg) for 21 days. (A) control gills (G0) show a normal histological appearance of primary and secondary lamellae. (B) GHg showing extensive lamellar thickening by severe hyperplasia (thin arrow) with stunted, fused, or complete lack of secondary lamellae. (C) GHg showing lamellar branchitis with moderate numbers of inflammatory cells, predominantly lymphocytes (thick arrow). (D) GN5+Hg shows gills apparently normal with occasional primary lamellar degeneration (thick arrow). (E) GN10+Hg shows focal tip lamellar hypertrophy (thin arrow) with mild lamellar sloughing (thick arrows). Image magnification = 400 ×
The intestine of the control group showed a normal histological appearance of the intestinal mucosa with scattered goblet cells (Fig. 6A). Meanwhile, extensive intestinal degenerative changes represented by diffuse, severe vacuolation were detected in GHg group (Fig. 6B). Moderate to severe enteritis were also seen characterized by moderate mucosal lymphocytic infiltrations extended to submucosal layers beside diffuse, mild to severe submucosal edema admixed with lymphocytes, and few eosinophilic granular cells (Fig. 6C and D). In contrast, the normal histological appearance of the intestine was partially restored, with insignificant differences in goblet cell numbers of GN5+Hg and GN10+Hg groups (Fig. 6E and F).
Histopathology of HgCl2 exposure in the intestine of Nile tilapia. The fish were fed with the control diet (G0) or exposed to 0.3 ppm HgCl2 and fed with the control diet (GHg), or with diets containing 5% N. oculata (GN5 + Hg) or 10% N. oculata (GN10+Hg) for 21 days. (A) G0 intestine shows normal histological appearance. (B) GHg shows diffuse, extensive intestinal swollen with multiple vacuolation (thin arrow). (C) GHg shows showing severe intestinal vacuolation (thin arrow) extensive submucosal edema (star) admixed with few eosinophilic granular cells (arrowhead). (D) GHg shows extensive enteritis represented by lymphocytic infiltrations of enterocytes and extended in the submucosal layer (arrow heads), which is mixed with edema and few RBCs (star). (E) GN5+Hg shows minimal submucosal leukocytic aggregations (thin arrow) with few lymphocytic and eosinophilic granular cells invading mucosal layer (arrowhead). (F) GN10+Hg shows diffuse moderate enterocyte vacuolation with few submucosal lymphocytic aggregations (thin arrow). Image magnification = 400 ×
The differential goblet cell numbers of intestinal sections were quantified between different treatment groups. There was a significant difference between groups (p < 0.000). Goblet cell number was significantly lower in the GHg group than in G0 (p < 0.000). In contrast, there is no significant difference between GN5+Hg and GN10+Hg compared to the G0 group (p < 0.991 and 0.070) (Figs. 4C and 7).
Histopathology of HgCl2 exposure in the intestinal goblet cells of Nile tilapia. The fish were fed with the control diet (G0) or exposed to 0.3 ppm HgCl2 and fed with the control diet (GHg), or with diets containing 5% N. oculata (GN5 + Hg) or 10% N. oculata (GN10+Hg) for 21 days. (A) G0 shows normal expression of goblet cells. (B) GHg shows low to a mild expression of goblet cells. (C) GN5+Hg shows similar goblet cells expression as the control. (D) GN10+Hg shows a moderate expression of goblet cells. Thin arrow = positive PAS cells. Image magnification = 400 ×
Discussion
Mercury (Hg2+) Toxicity
Heavy metals are a severe threat to the aquaculture environment causing chronic toxicity to the aquatics [38]. Therefore, exposure to sublethal dosages of these metals alarms and stimulates the fishes’ innate immune defense mechanisms differently. In an earlier study, the exposure to sublethal dosages from waterborne heavy metals elicited gilthead seabream (Sparus aurata) innate immunity. It increases mucus secretion from the skin, the most directly contacted organ with heavy metals [39]. Although some fish can detoxify Hg2+ after some exposure, others do not; this varies according to the fish species, developmental stage, and exposure dosage [40, 41]. The antioxidant activity of the large yellow croaker (Pseudosciaena crocea) juveniles was reported to be significantly elevated by their exposure to MeHg (4.0 µg L−1) for 30 days; this indicates the high antioxidant defense and innate immunity gained against the heavy metal contamination at the early life stages [42]. In an earlier study, Hg2+ toxicity was alleviated from the brain of peacock blennies fish ten days after exposure to a sublethal dose [43]. In contrast, Hg2+ was reported to induce chromosomal damage in wild fish (Dicentrarchus labrax L.), which doubled and continued this environmental disease for several contrasting seasons [44].
Feed Additive and Growth Performance
A nominal increase was observed in fish FW in the Hg2+ only group, possibly due to a hormetic effect, where a low toxicant dose could have a stimulatory effect [45]. However, the growth-suppressive effect of Hg2+ exposure was noticeable, where the feed additive N. oculata could not overcome the Hg2+ adverse effect. Still, at the same time, it was able to maintain growth within the level of the control one. Our results coincided with previous studies showing a direct relation between Hg2+ toxicity and fish growth reduction [46], which might owe to thyroid dysfunction, closely related to metabolism regulation and growth [38, 47]. Notably, the high protein content of N. oculata [48] added to feed in our experiment significantly enhanced the healthy fish’s growth (p < 0.05). It is known that proteins in tilapia feed improve their growth while increasing their nitrogenous wastes [49]. Noteworthy, the dietary N. oculata decreased the HSI without significance (p > 0.05) compared with the G0 group, indicating no adverse effect on fish health.
Mercury (Hg2+) Bioaccumulation
Our results revealed significantly (p < 0.05) higher levels of Hg2+ bioaccumulation in the muscles of all Hg2+ exposed groups, with a noticeable decrease in its level in the N. oculata-supplemented groups compared to the Hg2+ group. In addition, traces (0.04 mg/kg) were detected in the muscles of the unexposed fish; such a case is logical since Hg2 + can be created by the metabolism of the vapor of Hg0 or MeHg found naturally by small environmental levels [50]. Bioaccumulation of the Hg2+ is promoted by its absorption in biological systems after the deficiency of their antioxidant defense system [18]. In an earlier study, several routes (intraperitoneal injection, oral intubation, or semi-statical exposure) of short-term Hg2+ exposure at increasing doses caused dose-dependent Hg2+ accumulations and lesions of different severities in tilapia’s (Oreochromis niloticus) kidney, hepatopancreas, spleen, and intestine [24]. The relatively low Hg2+ concentration in muscle may be due to lower binding affinity with muscle proteins [51]. Similar findings were reported in other fish species exposed to HgCl2, including spotted snakehead (Channa punctata) [52], Japanese flounder (Paralichthys olivaceus) [53], juvenile zebra seabream (Diplodus cervinus, Lowe 1838) [54], and catfish (Heteropneustes fossilis) [23]. Noteworthy, because of its high content of phenolic and flavonoid compounds, N. oculata has a potential chelating effect on Hg2+ [55]. These compounds also have a variety of other beneficial biological properties, such as antioxidant and free radical scavenging activities. [56, 57]. Similarly, rohu (Labeo rohita) exposed to 0.12 mg/L Hg2+ for 21 days had their Hg2+ content reduced when fed a diet containing 2% Spirulina platensis (S. platensis) [30]. When applied at 7 and 10 percent, Amphora coffeaeformis reduced arsenic concentration in the muscle of African catfish exposed to arsenic at 38.3 mg/L for 15 days [58]. These findings suggest the potential role of N. oculata supplementation as a chelator of toxic metals in fish.
Serum Acetylcholinesterase (AChE) Activity
Acetylcholinesterase (AChE) activity is considered a valuable tool for recognizing frequent and continuous toxicant exposure [31]. AChE is an enzyme that controls and regulates the transmission of nerve impulses within the cholinergic synapsis; the higher the toxicity and metal ions bioaccumulation, the lower its enzymatic response [5]. Mainly, it catalyzes the hydrolysis process of the “acetylcholine” neurotransmitter into “choline” and “acetic acid,” which is a termination step after the activation prerequisite for neuronal transmission [59]. Our findings showed an improvement in the AChE activity of N. oculata dietary supplemented fish, more obviously in the GN10+Hg, after the significant decrease of its value elicited in the Hg2+ only group, which is an indicative factor for the detoxification efficacy of dietary N. oculata against the heavy metals’ bioaccumulation. Consistently, three carps, Labeo rohita, Catla, and Cirrhinus mrigala, revealed inhibition of the AChE in a dose-dependent manner when exposed to different concentrations of cadmium, zinc, and their combination [60]. Nile tilapia exposed to diazinon for 45 and 90 days revealed a significant decline in serum AChE that was slightly reversed by bentonite dietary supplementation [61].
Gene Expression Analysis
The Hg2+-only group in gills and intestines exhibited downregulation and upregulation of the relative expression of the targeted antioxidant enzyme-related genes (GSR and GPx) and the targeted stress-related genes (HSP and CAS), respectively. These findings are evident histopathologically, suggesting the harmful impact of Hg2+ bioaccumulation in fish tissues that reduces the production of the antioxidant enzyme, which leads to the persistence of oxidative stress. The primary detoxifying function of GPx and GSR is to stop the chain of radicals from propagating, preventing membrane oxidation and damage [62]. In our study, the downregulation of GPx and GSR transcripts during Hg2+ exposure could be due to increased utilization of oxidative enzymes for scavenging the H2O2 and hydroperoxide [63] or as a result of the direct impact of metal ions on the active motifs of the enzyme [64] or the exposed duration [65]. Our results are consistent with previous studies that frequently reported oxidative stress by multiple heavy metals exposure/bioaccumulation. Recently, Alam and his group conducted a study that reported sub-chronic toxicity to the Nile tilapia by dietary MeHg for 60 days, which alters the fish antioxidant status and downregulated the mRNA expression of GSR and GPx genes in the liver [46]. In a similar context, Hg2+ toxicity of HgCl2-exposed Nile tilapia under thermal stress conditions was marked by a reduction of the antioxidant enzymes, including GPx, in the serum, while a remarkable upregulation of the HSP-encoding gene was noticed in the lateral muscles [63]. More recently, arsenic (As) heavy metal exposure elevated the expression of stress-relevant genes, including HSP, in rohu carp fries [66].
On the contrary, the oxidative stress caused by exposure to MeHg for 30 days significantly elevated the GPx enzyme’s activity in juveniles’ large yellow croaker (Pseudosciaena crocea) [42]. Moreover, a dose-dependent upregulation was reported after Cr6+ exposure in expressing several oxidative stress-relevant genes, including the GSR gene, in the liver, owing to oxidative damage [67]. It is worth mentioning that the oxidative stress biomarkers and the degree of oxidative damage in different fish tissues might differ per the tissue of concern, fish age and sex, the dose of exposure, and the thermal conditions [63, 68].
The HSP proteins (HSPs) are essential for protein folding and safeguarding cells from stress; their production is linked to thermal stress caused by high temperatures and metals like cadmium, copper, and mercury [69]. The upregulation of HSP70 in this study by Hg2+ exposure in gills and intestines indicates its usefulness as a metal toxicity biomarker. In line with our findings, the juvenile large yellow croaker exhibited upregulation in the expression of HSP70 and HSP27 mRNA exposed to MeHg [42]. The upregulation of four heat shock proteins (HSP22, HSP90 beta, HSP90, and HSP70) was demonstrated in the transcriptomic analysis of Atlantic cod [70] and the liver of Nile tilapia during chronic exposure to chlorpyrifos (CPF) [32]. Similarly, heat stress and Hg2+ exposure can upregulate the expression levels of HSP70 in the muscle of Nile tilapia after 21 and 42 days of exposure [63].
To maintain equilibrium in living organisms, a family of conserved intracellular cysteine aspartate-specific proteases known as caspases are responsible for cell regulatory networks controlling inflammation and cell death [71]. In mammals, there are 14 molecules of the caspase protein family: caspases 1–3, 6–9, 12, and 14, which are found in both humans and mice, as well as caspases 4, 5, and 10 in humans (caspase 11 is found in mice) [72]. Initiator caspases (caspase-8 and -9) and executioner caspases (caspase-3, -6, and -7) are the two subclasses of caspases that are involved in apoptosis [71, 72]. Our study revealed an upregulation of the gills and intestines caspase3 mRNA level in the GHg and GN5+Hg; meanwhile, the GN10+Hg succeeded in maintaining its level to the control one. These results coincided with studies that demonstrated the effect of heavy metals on caspasce3 level, including fish Channa punctatus exposure to As or Hg [73] or rainbow trout gill-W1 cell lines [74] and testis of Gobius niger exposed to Cd [75].
Noteworthy, throughout our study, dietary N. oculata elevated the relative expression of the antioxidant enzymes-related genes and suppressed that of the stress protein-related genes. The 10% supplementing dose was more effective. This finding supports the efficacy of N. oculata feed supplementation for therapeutic applications against heavy metal contamination. N. oculata’s beneficial effects herein could be attributed to its biological constituents, as mentioned earlier, which play a crucial role in different aspects, one aspect as a metal chelator due to its phenolics and flavonoids [55, 76] and antioxidant components [56, 57]. Additionally, the surfaces of algal cells possess several functional groups, including hydroxyl, phosphoryl, amino, carboxyl, and sulphydryl (-SH) groups acting as adsorption sites responsible for heavy metals uptake. The other aspects as an immunomodulator owing to its high content of n3-long chain (LCPUFAs), mainly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), influencing the membrane fluidity of fish cells [77], lead to diminishing the inflammatory changes and augmenting the fish immune defense during [78,79,80].
In a similar context, several natural products, including algae, were incorporated into the fish diet and reported for protection against the toxicity of heavy metals, including mercury. Increased expression of hepatic and intestinal GST and GPx transcripts in Nile tilapia exposed to air stress has been consistently shown in studies using dietary microalgae like N. oculata [81]. During arsenic toxicity, feeding rainbow trout a diet containing Haematococcus pluvialis (H. pluvialis) extract improved cellular antioxidant defense by upregulating the expression of GPx, SOD, C, AT, and GSTA [82]. The hepatic antioxidative parameters SOD, GSH-Px, and GST gene levels were significantly reduced when Spirulina platensis was added to the diet, compared to the sodium sulfate intoxicated group [83]. Zahran et al. [32, 81] and Elabd et al. [84] showed that dietary supplementation with different microalgae positively upregulated the expression of the HSP70 gene in different organs, indicating the immunological properties of phyto-therapies. Moreover, N. oculata supplementation was able to diminish the caspase expression level to the control one, particularly in the GN10+Hg, which is consistent with Singh and his group demonstrating the potential role of ascorbic acid in lowering the caspase expression level in fish Channa punctatus exposed to As or Hg [85]. The same results were demonstrated in golden pompano fed on Odontella aurita microalga [86].
Histopathology
Our histopathological findings confirm the protective effect of an N. oculata-supplemented diet and support the results of the earlier analyzed biomarkers in the present study. Gills are highly affected as it is the most exposed organ to metal through continuous contact with water [87]. Marked pathological changes were observed in gills and intestinal sections exposed to Hg2+, represented by a lamellar fusion and inflammation, leading to a disturbance in gas exchange and decreased fish resistance. Our findings are similar to Nile tilapia gills damage following exposure to 0.03 mg/L HgCl2 [88] and Walking catfish (C. batrachus) intoxicated with 0.19 mg/L HgCl2 for 30 days [89]. The intestine represents a significant route of entry for a wide variety of toxicants present in the diet or in the water that the fish inhabit. In the same trend, the intestine of the exposed fish in GHg showed marked intestinal damage and inflammatory changes that lead to decreased absorptive capability, which are consistent with previous studies on seabream (Sparus aurata) and seabass (Dicentrarchus labrax) inhabiting the Bardawil Lagoon and exposed to different heavy metals, also on Channa punctatus and Dicentrarchus labrax exposed to mercury [90, 91], where the intestine showed fragmented, degenerated epithelium, lesions, and disarrangements of mucosal folding.
Interestingly, the gills and intestines of fish groups fed supplemented diets with low or high N. oculata exhibited no to mild pathological changes. The complete or partial restoration of tissue in these groups reflects the protective and regenerative capability of N. oculata. The absence of inflammation in the intestine and gills reflects the anti-inflammatory and antioxidant effect of N. oculata. These results are agreed with our previous studies that showed feeding N. oculata reduces the histopathology of Hg2+ on the liver, kidney, and gills and has an anti-inflammatory effect on Nile tilapia intestine and liver [26, 81].
Conclusion
In conclusion, mercuric intoxication in Nile tilapia leads to its accumulation in the musculature, inhibits acetylcholinesterase activity, downregulates genes related to antioxidants and stress, and elicits histopathological changes in the gills and intestine. Dietary supplementation with N. oculata effectively mitigated mercury’s harmful effects on fish health. In addition, our research provides strong support for establishing guidelines for the prevention, monitoring, and implementation of a cutting-edge biotech tool for managing aquatic pollution.
Data Availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
References
Malik D, Sharma AK, Sharma AK, Thakur R, Sharma M (2020) A review on impact of water pollution on freshwater fish species and their aquatic environment. Adv Environ Pollut Manag: Wastewater Impacts Treat Technol 1:10–28
Gray JE, Rimondi V, Costagliola P, Vaselli O, Lattanzi P (2014) Long-distance transport of Hg, Sb, and As from a mined area, conversion of Hg to methyl-Hg, and uptake of Hg by fish on the Tiber River Basin, west-central Italy. Environ Geochem Health 36(1):145–157
Davis J, Ross J, Bezalel S, Sim L, Bonnema A, Ichikawa G, Heim W, Schiff K, Eagles-Smith CA, Ackerman JT (2016) Hg concentrations in fish from coastal waters of California and Western North America. Sci Total Environ 568:1146–1156
Moiseenko T, Gashkina N (2020) Distribution and bioaccumulation of heavy metals (Hg, Cd and Pb) in fish: influence of the aquatic environment and climate. Environ Res Lett 15(11):115013
de Castro Rodrigues AP, Carvalheira RG, Gomes V, Arias ARL, Almosny NRP, Castilhos ZC, Bidone ED (2018) Acetylcholinesterase activity in fish exposed to mercury in Guanabara Bay, RJ. Brazil. Environmental Pollution and Protection 3(4):91–99
Azevedo LS, Pestana IA, da Costa Nery AF, Bastos WR, Souza CMM (2019) Variation in Hg accumulation between demersal and pelagic fish from Puruzinho Lake, Brazilian Amazon. Ecotoxicology 28(10):1143–1149
Charette T, Rosabal M, Amyot M (2021) Mapping metal (Hg, As, Se), lipid and protein levels within fish muscular system in two fish species (striped bass and northern pike). Chemosphere 265:129036
Gaxiola-Robles R, Labrada-Martagón V, Acosta-Vargas B, Méndez-Rodríguez LC, Zenteno-Savín T (2014) Interaction between mercury (Hg), arsenic (As) and selenium (Se) affects the activity of glutathione S-transferase in breast milk; possible relationship with fish and shellfish intake. Nutr Hosp 30(2):436–446
Hosseini M, Nabavi SMB, Nabavi SN, Pour NA (2015) Heavy metals (Cd Co, Cu, Ni, Pb, Fe, and Hg) content in four fish commonly consumed in Iran: risk assessment for the consumers. Environ Monit Assess 187(5):1–7
Hossain MB, Ahmed ASS, Sarker M, Islam S (2018) Human health risks of Hg, As, Mn, and Cr through consumption of fish, Ticto barb (Puntius ticto) from a tropical river, Bangladesh. Environ Sci Pollut Res 25(31):31727–31736
Zhang H, Feng X, Larssen T, Qiu G, Vogt RD (2010) In inland China, rice, rather than fish, is the major pathway for methylmercury exposure. Environ Health Perspect 118(9):1183–1188
Monteiro D, Rantin F, Kalinin A (2010) Inorganic mercury exposure: toxicological effects, oxidative stress biomarkers and bioaccumulation in the tropical freshwater fish matrinxã, Brycon amazonicus (Spix and Agassiz, 1829). Ecotoxicology 19(1):105–123
Caglayan C, Kandemir FM, Darendelioğlu E, Yıldırım S, Kucukler S, Dortbudak MB (2019) Rutin ameliorates mercuric chloride-induced hepatotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis. J Trace Elem Med Biol 56:60–68
Cardoso O, Puga S, Brandão F, Canário J, O’Driscoll NJ, Santos MA, Pacheco M, Pereira P (2017) Oxidative stress profiles in brain point out a higher susceptibility of fish to waterborne divalent mercury compared to dietary organic mercury. Mar Pollut Bull 122(1–2):110–121
Malqui H, Anarghou H, Merzouki M, Najimi M, Chigr F (2022) Effects of Mercury on General Homeostasis and Liver–Brain Interaction. In: Chatoui H, Merzouki M, Moummou H, Tilaoui M, Saadaoui, N, Brhich A (eds) Nutrition and Human Health. Springer: Cham. https://doi.org/10.1007/978-3-030-93971-7_17
Trivedi SP, Singh S, Trivedi A, Kumar M (2022) Mercuric chloride-induced oxidative stress, genotoxicity, haematological changes and histopathological alterations in fish Channa punctatus (B loch, 1793). J Fish Biol 100(4):868–883
Belger L, Forsberg BR (2006) Factors controlling Hg levels in two predatory fish species in the Negro river basin, Brazilian Amazon. Sci Total Environ 367(1):451–459
Lee J-H, Moniruzzaman M, Yun H, Lee S, Park Y, Bai SC (2016) Dietary vitamin C reduced mercury contents in the tissues of juvenile olive flounder (Paralichthys olivaceus) exposed with and without mercury. Environ Toxicol Pharmacol 45:8–14
Sathasivam R, Radhakrishnan R, Hashem A, Abd Allah EF (2019) Microalgae metabolites: a rich source for food and medicine. Saudi J Biol Sci 26(4):709–722
Sirakov I, Velichkova K, Stoyanova S, Staykov Y (2015) The importance of microalgae for aquaculture industry. Review. Int J Fish Aquat Stud 2(4):81–84
Roy SS, Pal R (2015) Microalgae in aquaculture: a review with special references to nutritional value and fish dietetics. Proc Zool Soc Springer 2015:1–8
Joseph J, Mwangi I, Swaleh S, Wanjau R, Ram M, Ngila J (2017) A comparative study of modified and unmodified algae (Pediastrum boryanum) for removal of lead, cadmium and copper in contaminated water. Water Qual 11:245–266
Kothari S, Choughule N (2015) Ameliorative stroke of selenium against toxicological effects of mercuric chloride in liver of freshwater catfish Heteropneustes fossilis (Bloch). Environ Toxicol 30(8):927–936
Kaewamatawong T, Rattanapinyopituk K, Ponpornpisit A, Pirarat N, Ruangwises S, Rungsipipat A (2013) Short-term exposure of Nile tilapia (Oreochromis niloticus) to mercury: histopathological changes, mercury bioaccumulation, and protective role of metallothioneins in different exposure routes. Toxicol Pathol 41(3):470–479
Al Ghais S, Bhardwaj V, Kumbhar P, Al Shehhi O (2019) Effect of copper nanoparticles and organometallic compounds (dibutyltin) on tilapia fish. J Basic Appl Zool 80(1):32
Mamdouh A-Z, Zahran E, Mohamed F, Zaki V (2021) Nannochloropsis oculata feed additive alleviates mercuric chloride-induced toxicity in Nile tilapia (Oreochromis niloticus). Aquat Toxicol 238:105936
Noga EJ (2010) Fish Disease: Diagnosis and Treatment. Wiley-Blackwell, Iowa, USA
National Research Council (NRC) (2011) Nutrient requirements of fish and shrimp. The National Academies Press: Washington, DC, USA
Zahran E, Awadin W, Risha E, Khaled AA, Wang T (2019) Dietary supplementation of Chlorella vulgaris ameliorates chronic sodium arsenite toxicity in Nile tilapia Oreochromis niloticus as revealed by histopathological, biochemical and immune gene expression analysis. Fish Sci 85(1):199–215
Shelke AD (2018) Effect of dietary spirulina platensis on metal distribution in Labeo rohita exposed to sublethal concentration of mercuric chloride. Trends Fish Res 7(3):111–115
Alves H, Silva A, Pavão J, Matos-Rocha T, Souza M, Costa J, Fonseca S, Pires L, Faé J, Santos A (2020) The acetylcholinesterase as indicative of intoxication for pesticide in farmers of conventional and organic cultivation. Braz J Biol 81:632–641
Zahran E, Elbahnaswy S, Risha E, El-Matbouli M (2020) Antioxidative and immunoprotective potential of Chlorella vulgaris dietary supplementation against chlorpyrifos-induced toxicity in Nile tilapia. Fish Physiol Biochem 46(4):1549–1560
Zahran E, El Sebaei MG, Awadin W, Elbahnaswy S, Risha E, Elseady Y (2020) Withania somnifera dietary supplementation improves lipid profile, intestinal histomorphology in healthy Nile tilapia (Oreochromis niloticus), and modulates cytokines response to Streptococcus infection. Fish Shellfish Immunol 106:133–141
Standen B, Peggs D, Rawling M, Foey A, Davies S, Santos G, Merrifield D (2016) Dietary administration of a commercial mixed-species probiotic improves growth performance and modulates the intestinal immunity of tilapia, Oreochromis niloticus. J Fish Shellfish Immunology 49:427–435
Roberts RJ (2012) Fish pathology. Wiley, Hoboken
Hoseini SM, Sinha R, Fazel A, Khosraviani K, Hosseinpour Delavar F, Arghideh M, Sedaghat M, Paolucci M, Hoseinifar SH, Van Doan H (2022) Histopathological damage and stress-and immune-related genes’ expression in the intestine of common carp, Cyprinus carpio exposed to copper and polyvinyl chloride microparticle. J Exp Zool Part A: Ecol Integr Physiol 337(2):181–190
Wang T, Gorgoglione B, Maehr T, Holland JW, Vecino JLG, Wadsworth S, Secombes CJ (2011) Fish suppressors of cytokine signaling (SOCS): gene discovery, modulation of expression and function. J Signal Transduct 20
Wang Y-J, Chen C-Z, Li P, Liu L, Chai Y, Li Z-H (2022) Chronic toxic effects of waterborne mercury on silver carp (Hypophthalmichthys molitrix) larvae. Water 14(11):1774
Guardiola FA, Dioguardi M, Parisi MG, Trapani MR, Meseguer J, Cuesta A, Cammarata M, Esteban MA (2015) Evaluation of waterborne exposure to heavy metals in innate immune defences present on skin mucus of gilthead seabream (Sparus aurata). Fish Shellfish Immunol 45(1):112–123
Shahjahan M, Taslima K, Rahman MS, Al-Emran M, Alam SI, Faggio C (2022) Effects of heavy metals on fish physiology–a review. Chemosphere 300
Suseno H (2015) Capability of catfish (Clarias gariepinus) to accumulate Hg2+ from water. Aceh Int J Sci Technol 4(3):93–98
Wu F, Huang W, Liu Q, Xu X, Zeng J, Cao L, Hu J, Xu X, Gao Y, Jia S (2018) Responses of antioxidant defense and immune gene expression in early life stages of large yellow croaker (Pseudosciaena crocea) under methyl mercury exposure. Front Physiol 9:1436
Naïja A, Kestemont P, Chénais B, Haouas Z, Blust R, Helal AN, Marchand J (2018) Effects of Hg sublethal exposure in the brain of peacock blennies Salaria pavo: molecular, physiological and histopathological analysis. Chemosphere 193:1094–1104
Mohmood I, Mieiro CL, Coelho JP, Anjum NA, Ahmad I, Pereira E, Duarte AC, Pacheco M (2012) Mercury-induced chromosomal damage in wild fish (Dicentrarchus labrax L.) reflecting aquatic contamination in contrasting seasons. Arch Environ Contam Toxicol 63(4):554–562
Biswas S, Bellare J (2021) Adaptive mechanisms induced by sparingly soluble mercury sulfide (HgS) in zebrafish: behavioural and proteomics analysis. Chemosphere 270:129438
Alam R, Abu Zeid E, Khalifa BA, Arisha AH, Reda RM (2021) Dietary exposure to methyl mercury chloride induces alterations in hematology, biochemical parameters, and mRNA expression of antioxidant enzymes and metallothionein in Nile tilapia. Environ Sci Pollut Res 28(24):31391–31402
Sun Y, Li Y, Liu Z, Chen Q (2018) Environmentally relevant concentrations of mercury exposure alter thyroid hormone levels and gene expression in the hypothalamic–pituitary–thyroid axis of zebrafish larvae. Fish Physiol Biochem 44(4):1175–1183
Zahran E, Elbahnaswy S, Ahmed F, Ibrahim I, Khaled AA, Eldessouki EA (2023) Nutritional and immunological evaluation of Nannochloropsis oculata as a potential Nile tilapia-aquafeed supplement. BMC Vet Res 19(1):65
Qiang J, He J, Yang H, Wang H, Kpundeh M, Xu P, Zhu Z (2014) Temperature modulates hepatic carbohydrate metabolic enzyme activity and gene expression in juvenile GIFT tilapia (Oreochromis niloticus) fed a carbohydrate-enriched diet. J Therm Biol 40:25–31
Clarkson TW, Magos L (2006) The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36(8):609–662
Osman A (2012) Biomarkers in Nile Tilapia Oreochromis niloticus niloticus (Linnaeus, 1758) to assess the impacts of river Nile pollution: bioaccumulation, biochemical and tissues biomarkers. J Environ Prot 3(8A):966–977. https://doi.org/10.4236/jep.2012.328112
Arya A, Sharma GD (2015) Combined effects of cadmium and mercury on some biochemical and histochemical changes in liver, kidney and gills of Channa punctatus (Bloch). Int J Pharm Pharm Sci 7(8):117–120
Huang W, Cao L, Ye Z, Lin L, Chen Q, Dou S (2012) Tissue-specific bioaccumulation and oxidative stress responses in juvenile Japanese flounder (Paralichthys olivaceus) exposed to mercury. Chin J Oceanol Limnol 30(4):569–579
Branco V, Canário J, Lu J, Holmgren A, Carvalho C (2012) Mercury and selenium interaction in vivo: effects on thioredoxin reductase and glutathione peroxidase. Free Radical Biol Med 52(4):781–793
Ebrahimzadeh MA, Khalili M, Dehpour AA (2018) Antioxidant activity of ethyl acetate and methanolic extracts of two marine algae, Nannochloropsis oculata and Gracilaria gracilis-an in vitro assay. Braz J Pharm Sci 54(1). https://doi.org/10.1590/s2175-97902018000117280
Li H-B, Cheng K-W, Wong C-C, Fan K-W, Chen F, Jiang Y (2007) Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem 102(3):771–776
Fernando IS, Kim M, Son K-T, Jeong Y, Jeon Y-J (2016) Antioxidant activity of marine algal polyphenolic compounds: a mechanistic approach. J Med Food 19(7):615–628
Mekkawy IA, Mahmoud UM, Moneeb RH, Sayed AE-DH (2020) Significance assessment of Amphora coffeaeformis in arsenic-induced hemato-biochemical alterations of African catfish (Clarias gariepinus). Front Mar Sci 7:191
Cheng B-N, Jin Y-L, Chen B-Q, Zhu L-Y, Xu Z-C, Shen T (2016) Serum cholinesterase: a potential assistant biomarker for hand, foot, and mouth disease caused by enterovirus 71 infection. Infect Dis Poverty 5(1):1–5
Rani S, Gupta R, Rani M (2015) Heavy metal induced toxicity in fish with special reference to zinc and cadmium. Int J Fish Aquat Stud 3(2):118–123
Abbas EA, Mowafy RE, Khalil AA, Sdeek FA (2021) The potential role of the dietary addition of bentonite clay powder in mitigating diazinon-induced hepatorenal damage, oxidative stress, and pathological alterations in Nile tilapia. Aquaculture 533:736182
Lee KH, Cha M, Lee BH (2020) Neuroprotective effect of antioxidants in the brain. Int J Mol Sci 21(19):7152
Waheed R, El Asely AM, Bakery H, El-Shawarby R, Abuo-Salem M, Abdel-Aleem N, Malhat F, Khafaga A, Abdeen A (2020) Thermal stress accelerates mercury chloride toxicity in Oreochromis niloticus via up-regulation of mercury bioaccumulation and HSP70 mRNA expression. Sci Total Environ 718:137326
Zahran E, Risha E (2014) Modulatory role of dietary Chlorella vulgaris powder against arsenic-induced immunotoxicity and oxidative stress in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 41(2):654–662
Huo H, Wang S, Bai Y, Liao J, Li X, Zhang H, Han Q, Hu L, Pan J, Li Y (2021) Copper exposure induces mitochondrial dynamic disorder and oxidative stress via mitochondrial unfolded protein response in pig fundic gland. Ecotoxicol Environ Saf 223:112587
Rabbane MG, Kabir MA, Habibullah-Al-Mamun M, Mustafa MG (2022) Toxic effects of arsenic in commercially important fish rohu carp, Labeo rohita of Bangladesh. Fishes 7(5):217
Awasthi Y, Ratn A, Prasad R, Kumar M, Trivedi SP (2018) An in vivo analysis of Cr6+ induced biochemical, genotoxicological and transcriptional profiling of genes related to oxidative stress, DNA damage and apoptosis in liver of fish, Channa punctatus (Bloch, 1793). Aquat Toxicol 200:158–167
Miller LL, Isaacs MA, Martyniuk CJ, Munkittrick KR (2015) Using molecular biomarkers and traditional morphometric measurements to assess the health of slimy sculpin (Cottus cognatus) from streams with elevated selenium in North-Eastern British Columbia. Environ Toxicol Chem 34(10):2335–2346
Rajeshkumar S, Munuswamy N (2011) Impact of metals on histopathology and expression of HSP 70 in different tissues of milk fish (Chanos chanos) of Kaattuppalli Island, South East Coast, India. Chemosphere 83(4):415–421
Yadetie F, Karlsen OA, Lanzén A, Berg K, Olsvik P, Hogstrand C, Goksøyr A (2013) Global transcriptome analysis of Atlantic cod (Gadus morhua) liver after in vivo methylmercury exposure suggests effects on energy metabolism pathways. Aquat Toxicol 126:314–325
Li S, Li J, Peng W, Hao G, Sun J (2019) Characterization of the responses of the caspase 2, 3, 6 and 8 genes to immune challenges and extracellular ATP stimulation in the Japanese flounder (Paralichthys olivaceus). BMC Vet Res 15(1):1–12
McIlwain DR, Berger T, Mak TW (2013) Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 5(4):a008656
Morcillo P, Cordero H, Meseguer J, Esteban MÁ, Cuesta A (2015) In vitro immunotoxicological effects of heavy metals on European sea bass (Dicentrarchus labrax L.) head-kidney leucocytes. Fish Shellfish Immunol 47(1):245–254
Krumschnabel G, Ebner HL, Hess MW, Villunger A (2010) Apoptosis and necroptosis are induced in rainbow trout cell lines exposed to cadmium. Aquat Toxicol 99(1):73–85
Migliarini B, Campisi AM, Maradonna F, Truzzi C, Annibaldi A, Scarponi G, Carnevali O (2005) Effects of cadmium exposure on testis apoptosis in the marine teleost Gobius niger. Gen Comp Endocrinol 142(1):241–247
Custódio L, Soares F, Pereira H, Rodrigues MJ, Barreira L, Rauter AP, Alberício F, Varela J (2015) Botryococcus braunii and Nannochloropsis oculata extracts inhibit cholinesterases and protect human dopaminergic SH-SY5Y cells from H2O2-induced cytotoxicity. J Appl Phycol 27(2):839–848
Snyder RJ, Hennessey TM (2003) Cold tolerance and homeoviscous adaptation in freshwater alewives (Alosa pseudoharengus). Fish Physiol Biochem 29(2):117–126
Wall R, Ross RP, Fitzgerald GF, Stanton C (2010) Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. J Nutrition Rev 68(5):280–289
Calder PC (2017) Omega-3 fatty acids and inflammatory processes: from molecules to man. J Biochem Soc Trans 45(5):1105–1115
Buoite Stella A, Gortan Cappellari G, Barazzoni R, Zanetti M (2018) Update on the impact of omega 3 fatty acids on inflammation, insulin resistance and sarcopenia: a review. J Int J Mol Sci 19(1):218
Zahran E, Elbahnaswy S, Ibrahim I, Khaled AA (2021) Nannochloropsis oculata enhances immune response, transcription of stress, and cytokine genes in Nile tilapia subjected to air exposure stress. Aquac Rep 21:100911
Milan FS, Maleki BRS, Moosavy M-H, Mousavi S, Sheikhzadeh N, Khatibi SA (2021) Ameliorating effects of dietary Haematococcus pluvialis on arsenic-induced oxidative stress in rainbow trout (Oncorhynchus mykiss) fillet. Ecotoxicol Environ Saf 207:111559
Awed EM, Sadek KM, Soliman MK, Khalil RH, Younis EM, Abdel-Warith A-WA, Van Doan H, Dawood MA, Abdel-Latif HM (2020) Spirulina platensis alleviated the oxidative damage in the gills, liver, and kidney organs of Nile tilapia intoxicated with sodium sulphate. Animals 10(12):2423
Elabd H, Wang H-P, Shaheen A, Matter A (2020) Nano spirulina dietary supplementation augments growth, antioxidative and immunological reactions, digestion, and protection of Nile tilapia, Oreochromis niloticus, against Aeromonas veronii and some physical stressors. Fish Physiol Biochem 46(6):2143–2155
Singh S, Srivastava A, Allen T, Bhagat N, Singh N (2020) Identification of heavy metal toxicity–induced biomarkers and the protective tole of ascorbic acid supplementation in Channa punctatus. Int J Pharm Sci Res 11:1098–1109
Zhao W, Yao R, He X-S, Liao Z-H, Liu Y-T, Gao B-Y, Zhang C-W, Niu J (2022) Beneficial contribution of the microalga Odontella aurita to the growth, immune response, antioxidant capacity, and hepatic health of juvenile golden pompano (Trachinotus ovatus). Aquaculture 555:738206
Chavan V, Muley D (2014) Effect of heavy metals on liver and gill of fish Cirrhinus mrigala. Int J Curr Microbiol App Sci 3(5):277–288
Jasim MA, Sofian-Azirun M, Yusoff I, Rahman MM (2016) Bioaccumulation and histopathological changes induced by toxicity of mercury (HgCl2) to tilapia fish Oreochromis niloticus. Sains Malays 45(1):119–127
Selvanathan J, Vincent S, Nirmala A (2013) Histopathology changes in freshwater fish Clarias batrachus (Linn.) exposed to mercury and cadmium. Int J Life Sci Pharma Res 3(2):11–21
Begam M, Sengupta M (2013) Effects of mercury on the activities of antioxidant defences in intestinal macrophages of fresh water teleost Channa punctatus (Bloch 1793). Int J Fish Aquat Stud 2:172–179
Giari L, Simoni E, Manera M, Dezfuli B (2008) Histo-cytological responses of Dicentrarchus labrax (L.) following mercury exposure. Ecotoxicol Environ Saf 70(3):400–410
NRC N (2011) Nutrient requirements of fish and shrimp. In.: The National Academies Press Washington, DC, USA: 376
Acknowledgements
The authors are grateful to the Aquatic Animal Medicine Laboratory, Faculty of Veterinary Medicine, Mansoura University, for in-kind support for the present study.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
EZ: conceptualization, validation, formal analysis, supervision, and writing—reviewing and editing. FA: investigation, methodology, and writing—original draft. ZH: methodology and contribute to writing—original draft. II: methodology and contribution to writing—original draft. AAK: investigation, methodology, and resources. DP: reviewing and editing. MGE: investigation, methodology, and resources.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical Approval
The current study was conducted at the experimental facility of the AAM Laboratory, Faculty of Veterinary Medicine, Mansura University, Mansoura, Egypt. Procedures for animal care and management were approved by the Ethical Research Committee of Mansoura University, Code number: MU-ACUC (VM.R.23.01.42).
Consent to Participate
Not applicable.
Consent for Publication
The manuscript has never been published in other journals.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zahran, E., Ahmed, F., Hassan, Z. et al. Toxicity Evaluation, Oxidative, and Immune Responses of Mercury on Nile Tilapia: Modulatory Role of Dietary Nannochloropsis oculata. Biol Trace Elem Res 202, 1752–1766 (2024). https://doi.org/10.1007/s12011-023-03771-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03771-4