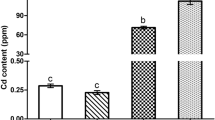
Abstract
Cadmium (Cd) can damage tissues by inducing oxidative stress, lymphocyte infiltration, and inflammation in these sites. Meanwhile, astilbin (Ast) is an antioxidant agent. At present, only a few mechanisms of Cd-induced adipose tissue damage have been described. Herein, we assessed the potential protective effects and the molecular mechanism underlying the antioxidant properly of Ast after Cd intake in chicken adipose tissue. In this study, a total of 160 7-day-old roosters were randomly divided into four groups. Roosters were fed with a basic diet (C group), Ast 40 mg/kg (Ast group), CdCl2 150 mg/kg + Ast 40 mg/kg (Cd/Ast group), and CdCl2 150 mg/kg (Cd group) for 60 days. We found that Cd intake changed the morphology and structure of adipose tissues and decreased the expression of several antioxidants, including total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), catalase (CAT), and total antioxidant capacity (T-AOC), but increased those of oxidative stress markers including malondialdehyde (MDA), inducible nitric oxide synthase (iNOS), NO, and H2O2. Cd further activated the nuclear factor kappa B (NF-κB) signaling pathway and increased the expression of the inflammation-related mediators, interleukin 1beta (IL-1β), interleukin 6 (IL-6), interleukin 8 (IL-8), interleukin 10 (IL-10), cyclooxygenase-2 (COX-2), iNOS, prostaglandin E synthase (PTGES), tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ). Cd-induced oxidative stress upregulated the expression of three heat shock proteins (HSPs), including HSP27, HSP70, and HSP90. Summarily, Cd causes oxidative stress-mediated tissue damage by activating the NF-κB pathway, promoting inflammation and upregulating the expression of HSPs. However, Ast supplementation modulates oxidative stress in adipose tissue by inhibiting inflammation mediated by the NF-κB pathway and regulating the expression of HSPs.
Graphical abstract







Similar content being viewed by others
Data Availability
The authors declare that the data supporting the findings of this study are available within the article.
Abbreviations
- Cd:
-
Cadmium
- Ast:
-
Astilbin
- cDNA:
-
Complementary DNA
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- HSPs:
-
heat shock proteins
- NF-κB:
-
Nuclear factor kappa B
- TBST:
-
Tris-buffered saline-Tween 20
- IL-1β:
-
Interleukin 1beta
- IL-6:
-
Interleukin 6
- IL-8:
-
Interleukin 8
- IL-10:
-
Interleukin 10
- TNF-α:
-
Tumor necrosis factor-alpha
- IFN-γ:
-
Interferon-gamma
- COX-2:
-
Cycooxygenase-2
- iNOS:
-
Inducible nitric oxide synthase
- T-SOD:
-
Total superoxide dismutase
- GSH-Px:
-
Glutathione peroxidase
- CAT:
-
Catalase
- T-AOC:
-
Total antioxidant capacity
- MDA:
-
Malondialdehyde
- PTGES:
-
Prostaglandin E synthase
References
Fay MJ, Alt LAC, Ryba D, Salamah R, Peach R, Papaeliou A, Zawadzka S et al. (2018) Cadmium nephrotoxicity is associated with altered microRNA expression in the rat renal cortex. Toxics 16 https://doi.org/10.3390/toxics6010016
Gobe G, Crane D (2010) Mitochondria, reactive oxygen species and cadmium toxicity in the kidney. Toxics 16 https://doi.org/10.1016/j.toxlet.2010.04.013
Le QG, Ishiwata-Kimata Y, Kohno K, Kimata Y (2016) Cadmium impairs protein folding in the endoplasmic reticulum and induces the unfolded protein response. FEMS Yeast Res, fow049 https://doi.org/10.1093/femsyr/fow049
Liu F, Wang XY, Zhou XP, Li ZP, Song XB, Wang ZY, et al. (2017) Cadmium disrupts autophagic flux by inhibiting cytosolic Ca2+-dependent autophagosome-lysosome fusion in primary rat proximal tubular cells. Toxicology 13-23 https://doi.org/10.1016/j.tox.2017.03.016
Chen X, Bi M, Yang J, Cai J, Zhang H, Zhu Y et al. (2021) Cadmium exposure triggers oxidative stress, necroptosis, Th1/Th2 imbalance and promotes inflammation through the TNF-α/NF-κB pathway in swine small intestine. J Hazard Mater 421:126704. https://doi.org/10.1016/j.jhazmat.2021.126704
Saely CH, Geiger K, Drexel H (2012) Brown versus white adipose tissue: a mini-review. Gerontology 15-23 https://doi.org/10.1159/000321319
Djokic J, Ninkov M, Mirkov I, Popov Aleksandrov A, Zolotarevski L, Kataranovski D, et al. (2014) Differential effects of cadmium administration on peripheral blood granulocytes in rats. Environ Toxicol Pharmacol 210-219 https://doi.org/10.1016/j.etap.2013.11.026
Liu J, Qu W, Kadiiska MB (2009) Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol Appl Pharmacol 209-214 https://doi.org/10.1016/j.taap.2009.01.029
Rani A, Kumar A, Lal A, Pant M (2014) Cellular mechanisms of cadmium-induced toxicity: a review. Int J Environ Health Res 378–399 https://doi.org/10.1080/09603123.2013.835032
Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K (2016) Redox-and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol 1-37 https://doi.org/10.1007/s00204-015-1579-5
Nair AR. Degheselle O, Smeets K, Van Kerkhove E, Cuypers A (2013) Cadmium-induced pathologies: where is the oxidative balance lost (or not)? Int J Mol Sci 6116-6143. https://doi.org/10.3390/ijms14036116
Zheng Y, Guan H, Yang J, Cai J, Liu Q, Zhang Z (2021) Calcium overload and reactive oxygen species accumulation induced by selenium deficiency promote autophagy in swine small intestine. Anim Nutr J 997-1008. https://doi.org/10.1016/j.aninu.2021.05.005
Cao H, Gao F, Xia B, Zhang M, Liao Y, Yang Z, et al. (2016) Alterations in trace element levels and mRNA expression of Hsps and inflammatory cytokines in livers of duck exposed to molybdenum or/and cadmium. Ecotoxicol Environ Saf 93-101 https://doi.org/10.1016/j.ecoenv.2015.12.003
Mitchell JP, Carmody RJ (2018) NF-κB and the transcriptional control of inflammation. Int Rev Cell Mol Biol 41-84 https://doi.org/10.1016/bs.ircmb.2017.07.007
Sen R, Baltimore D (1986) Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell 921-928 https://doi.org/10.1016/0092-8674(86)90807-x
Bonizzi G, Karin M (2004) The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol 280-288 https://doi.org/10.1016/j.it.2004.03.008
Baltimore, D (2011) NF-κB is 25. Nat Immunol 683-685.https://doi.org/10.1038/cr.2011.13
Schreck R, Albermann K, Baeuerle, PA (1992) Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radic Res Commun 221-237 https://doi.org/10.3109/10715769209079515
Pahl HL (1999) Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 6853-6866. https://doi.org/10.1038/sj.onc.1203239
Luo JL, Maeda S, Hsu LC, Yagita H, Karin M (2004) Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell 297-305. https://doi.org/10.1016/j.ccr.2004.08.012
Neurath MF, Becker C, (1998) Barbulescu, K. Role of NF-kappaB in immune and inflammatory responses in the gut. Gut 856-860 https://doi.org/10.1136/gut.43.6.856
Calderwood SK, Mambula SS, Gray PJ Jr, Theriault JR (2007) Extracellular heat shock proteins in cell signaling. FEBS Lett 3689-3694. https://doi.org/10.1016/j.febslet.2007.04.044
Noble EG, Milne KJ, Melling CW (2008) Heat shock proteins and exercise: a primer. Appl Physiol Nutr Metab 1050-1065 https://doi.org/10.1139/H08-069
Rogers RS, Beaudoin MS, Wheatley JL, Wright DC, Geiger PC (2015) Heat shock proteins: in vivo heat treatments reveal adipose tissue depot-specific effects. J Appl Physiol 98-106 https://doi.org/10.1152/japplphysiol.00286.2014
Wang Y, Liu J, Chen R, Qi M, Tao D, Xu S. The antagonistic effects of selenium yeast (sey) on cadmium-induced inflammatory factors and the heat shock protein expression levels in chicken livers. Biol Trace Elem Res (2020) https://doi.org/10.1007/s12011-020-02039-5
Hua S, Zhang Y, Liu, J, Dong L, Huang J, et al. (2018) Ethnomedicine, phytochemistry and pharmacology of Smilax glabra: an important traditional Chinese medicine. Am J Chin Med 261-297 https://doi.org/10.1142/S0192415X18500143
Naqinezhad A, Nabavi SM, Nabavi SF, Ebrahimzadeh MA (2012) Antioxidant and antihemolytic activities of flavonoid rich fractions of Artemisia tschernieviana Besser. Eur Rev Med Pharmacol Sci 16 Suppl 88-94. PMID: 22957422
Petacci F, Freitas SS, Brunetti IL, Khalil NM (2010) Inhibition of peroxidase activity and scavenging of reactive oxygen species by astilbin isolated from Dimorphandra mollis (Fabaceae, Caesalpinioideae). Biol Res 63–74. PMID: 21157633
Li GS, Jiang WL, Yue XD, Qu GW, Tian JW, Wu J, et al. (2009) Effect of astilbin on experimental diabetic nephropathy in vivo and in vitro. Planta Med 470-475 https://doi.org/10.1055/s-0029-1185802
Ding Y, Liang Y, Deng B, Qiao A, Wu K, Xiao W, et al. (2014) Induction of TGF-β and IL-10 production in dendritic cells using astilbin to inhibit dextran sulfate sodium-induced colitis. Biochem Biophys Res Commun 529-534 https://doi.org/10.1016/j.bbrc.2014.02.136
Wang SW, Xu Y, Weng YY, Fan XY, Bai YF, Zheng XY et al. (2018) Astilbin ameliorates cisplatin-induced nephrotoxicity through reducing oxidative stress and inflammation. Food Chem Toxicol 227:236. https://doi.org/10.1016/j.fct.2018.02.041
Wang M, Zhao J, Zhang N, Chen J (2016) Astilbin improves potassium oxonate-induced hyperuricemia and kidney injury through regulating oxidative stress and inflammation response in mice. Biomed Pharmacother 975:988. https://doi.org/10.1016/j.biopha.2016.07.025
Li Y, Wang R, Xue L, Yang Y, Zhi F (2020) Astilbin protects against cerebral ischaemia/reperfusion injury by inhibiting cellular apoptosis and ROS-NLRP3 inflammasome axis activation. Int Immunopharmacol 106571 https://doi.org/10.1016/j.intimp.2020.106571
Diao H, Kang Z, Han F, Jiang W (2013) Astilbin protects diabetic rat heart against ischemia-reperfusion injury via blockade of HMGB1-dependent NF-κB signaling pathway. Food Chem Toxicol 104:110. https://doi.org/10.1016/j.fct.2013.10.045
Wang J, Zhao Y, Xu Q (2004) Astilbin prevents concanavalin A-induced liver injury by reducing TNF-alpha production and T lymphocytes adhesion. J Pharm Pharmacol 495:502
Li T, Yu H, Song Y, Zhang R, Ge M (2019) Protective effects of Ganoderma triterpenoids on cadmium-induced oxidative stress and inflammatory injury in chicken livers. J Trace Elem Med Biol 118–125. https://doi.org/10.1016/j.jtemb.2018.12.010
Okereafor U, Makhatha M, Mekuto L, Uche-Okereafor N, Sebola T, Mavumengwana V (2020) Toxic metal implications on agricultural soils, plants, animals, aquatic life and human health. Int J Environ Res Public Health 2204. https://doi.org/10.3390/ijerph17072204
Xin C, Guangliang S, Qing Z, Qingqing L, Hang Y, Yiming Z, et al. (2020) Astilbin protects chicken peripheral blood lymphocytes from cadmium-induced necroptosis via oxidative stress and the PI3K/Akt pathway. Ecotoxicol Environ Saf 110064. https://doi.org/10.1016/j.ecoenv.2019.110064
Hu X, Zhang R, Xie Y, Wang H, Ge M (2017) The protective effects of polysaccharides from Agaricus blazei Murill against cadmium-induced oxidant stress and inflammatory damage in chicken livers. Biol Trace Elem Res 117–126. https://doi.org/10.1007/s12011-016-0905-y
Moynihan M, Telléz-Rojo MM, Colacino J, Jones A, Song PXK, Cantoral A et al. (2019) Prenatal cadmium exposure is negatively associated with adiposity in girls not boys during adolescence. Front Public Health. https://doi.org/10.3389/fpubh.2019.00061
Kurien BT, Scofield RH (2006) Western blotting. Methods 2015:283–293. https://doi.org/10.1007/978-1-4939-2694-7_5
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 402–408. https://doi.org/10.1006/meth.2001.1262
Abd El-Hack ME, Abdelnour SA, Abd El-Moneim AEE, Arif M, Khafaga A, Shaheen H et al. (2019) Putative impacts of phytogenic additives to ameliorate lead toxicity in animal feed. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-019-05805-8
Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A (2020) The effects of cadmium toxicity. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph17113782
Tinkov AA, Gritsenko VA, Skalnaya MG, Cherkasov SV, Aaseth J, Skalny AV (2018) Gut as a target for cadmium toxicity. Environ Pollut. https://doi.org/10.1016/j.envpol.2017.12.114
Tinkov AA, Filippini T, Ajsuvakova OP, Skalnaya MG, Aaseth J, Bjørklund G et al. (2018) Cadmium and atherosclerosis: a review of toxicological mechanisms and a meta-analysis of epidemiologic studies. Environ Res. https://doi.org/10.1016/j.envres.2018.01.008
Kawakami T, Sugimoto H, Furuichi R, Kadota Y, Inoue M, Setsu K, et al. (2010) Cadmium reduces adipocyte size and expression levels of adiponectin and Peg1/Mest in adipose tissue. Toxicology 20-26 https://doi.org/10.1016/j.tox.2009.07.022
Nemmiche S (2017) Oxidative signaling response to cadmium exposure. Toxicol Sci 4:10. https://doi.org/10.1093/toxsci/kfw222
Hou L, Liu S, Zhao C, Fan L, Hu H, Yin S (2021) The combination of T-2 toxin and acrylamide synergistically induces hepatotoxicity and nephrotoxicity via the activation of oxidative stress and the mitochondrial pathway. Toxicon 65-72. https://doi.org/10.1016/j.toxicon.2020.11.007
Li X, Ge M, Zhu W. Wang P, Wang J, Tai T et al. (2021) Protective effects of astilbin against cadmium-induced apoptosis in chicken kidneys via endoplasmic reticulum stress signaling pathway. Biol Trace Elem Res https://doi.org/10.1007/s12011-021-03029-x
Mesalam NM, Aldhumri SA, Gabr SA, Ibrahim MA, Al-Mokaddem AK, Abdel-Moneim AE (2021) Putative abrogation impacts of Ajwa seeds on oxidative damage, liver dysfunction and associated complications in rats exposed to carbon tetrachloride. Mol Biol Rep https://doi.org/10.1007/s11033-021-06544-1
Lazarus M, Orct T, Aladrović J, Ljubić BB, Jurasović J, Blanuša M (2011) Effect of selenium pre-treatment on antioxidative enzymes and lipid peroxidation in Cd-exposed suckling rats. Biol Trace Elem Res 611:622. https://doi.org/10.1007/s12011-010-8775-1
Zhuang J, Nie G, Yang F, Dai X, Cao H, Xing, C et al. (2019) Cadmium induces cytotoxicity through oxidative stress-mediated apoptosis pathway in duck renal tubular epithelial cells. Toxicol In Vitro 104625 https://doi.org/10.1016/j.tiv.2019.104625
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 529:539. https://doi.org/10.2174/1568026013394831
Klaassen CD, Liu J (1997) Role of metallothionein in cadmium-induced hepatotoxicity and nephrotoxicity. Drug Metab Rev 79:102. https://doi.org/10.3109/03602539709037574
Kim S, Cheon HS, Kim SY, Juhnn YS, Kim YY (2013) Cadmium induces neuronal cell death through reactive oxygen species activated by GADD153. BMC Cell Biol4. https://doi.org/10.1186/1471-2121-14-4
Sharma A, Gupta S, Chauhan S, Nair A, Sharma P (2020) Astilbin: a promising unexplored compound with multidimensional medicinal and health benefits. Pharmacol Res https://doi.org/10.1016/j.phrs.2020.104894
Abdel-Moneim AE, Shehata AM, Alzahrani SO, Shafi ME, Mesalam NM, Taha AE, et al. (2020) The role of polyphenols in poultry nutrition. J Anim Physiol Anim Nutr (Berl) https://doi.org/10.1111/jpn.13455
Vallabhapurapu S, Karin M (2009) Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol 693:733. https://doi.org/10.1146/annurev.immunol.021908.132641
Napetschnig J, Wu H (2013) Molecular basis of NF-κB signaling. Annu Rev Biophys 443:68. https://doi.org/10.1146/annurev-biophys-083012-130338
Morgan MJ, Liu ZG (2011) Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res 103:115. https://doi.org/10.1038/cr.2010.178
Abravaya K, Myers MP, Murphy SP, Morimoto RI (1992) The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev 1153:1164. https://doi.org/10.1101/gad.6.7.1153
Baler R, Welch WJ, Voellmy R (1992) Heat shock gene regulation by nascent polypeptides and denatured proteins: hsp70 as a potential autoregulatory factor. J Cell Biol 1151:1159. https://doi.org/10.1083/jcb.117.6.1151
Okada M, Hasebe N, Aizawa Y, Izawa K, Kawabe J, Kikuchi K (2004) Thermal treatment attenuates neointimal thickening with enhanced expression of heat-shock protein 72 and suppression of oxidative stress. Circulation 1763:1768. https://doi.org/10.1161/01.CIR.0000124226.88860.55
Morimoto RI (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev 3788:3796. https://doi.org/10.1101/gad.12.24.3788
Yoo CG, Lee S, Lee CT, Kim YW, Han SK, Shim YS (2000) Anti-inflammatory effect of heat shock protein induction is related to stabilization of I kappa B alpha through preventing I kappa B kinase activation in respiratory epithelial cells. J Immunol 5416:5423. https://doi.org/10.4049/jimmunol.164.10.5416
Pritts TA, Wang Q, Sun X, Fischer DR, Hungness ES, Fischer JE et al. (2002) The stress response decreases NF-kappaB activation in liver of endotoxemic mice. Shock 33:37. https://doi.org/10.1097/00024382-200207000-00007
Liu Y, Yu M, Cui J, Du Y, Teng X, Zhang Z (2021) Heat shock proteins took part in oxidative stress-mediated inflammatory injury via NF-κB pathway in excess manganese-treated chicken livers. Ecotoxicol Environ Saf 226:112833. https://doi.org/10.1016/j.ecoenv.2021.112833
Malhotra V, Wong HR (2002) Interactions between the heat shock response and the nuclear factor-kappa B signaling pathway. Crit Care Med 30(1 Suppl):S89–95. https://pubmed.ncbi.nlm.nih.gov/11782566/
Acknowledgements
We thank the members of the Chinese veterinary laboratory at the College of Veterinary Medicine, Northeast Agricultural University, for helping us to collect the samples. Our sincere gratitude to the Heilongjiang Key Laboratory for Laboratory Animals and Comparative Medicine.
Funding
This study was funded by the National Natural Science Foundation of China (No.31702282), the “Academic backbone” Project of Northeast Agricultural University (No. 20XG32), the University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (UNPYSCT-2016139), the Northeast Agricultural University Student Innovation Practical Training project (No. 202010224076), the “Young Talents” Project of Northeast Agricultural University (No.18QC41), and the Northeast Agricultural University/Scientific Observing and Experimental Station of Animal Nutrition and Feed Science in Northeast, Ministry of Agriculture. P.R. China (yy-2017–09).
Author information
Authors and Affiliations
Contributions
Jianxu Sun: conceptualization, data curation, writing-original draft. Zitao Jiao: searching literature, writing-original draft. Xiuyu Li: experiment. Weifeng Zhu: Experiment. Panpan Wang: experiment. Jiangfeng Wang: experiment. Tiange Tai: experiment. Yuxi Wang: experiment. Haibin Wang: writing-Reviewing. Guangliang Shi: methodology, financial support, writing-reviewing.
Corresponding author
Ethics declarations
Ethics approval
All procedures used in this study were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University. This article does not contain any studies with human performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, J., Jiao, Z., Zhu, W. et al. Astilbin Attenuates Cadmium-Induced Adipose Tissue Damage by Inhibiting NF-κB Pathways and Regulating the Expression of HSPs in Chicken. Biol Trace Elem Res 201, 2512–2523 (2023). https://doi.org/10.1007/s12011-022-03327-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03327-y