Abstract
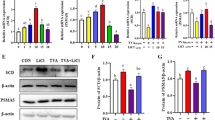
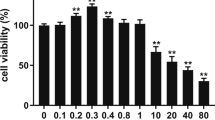
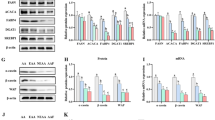
Lithium is one of the trace elements with many physiological properties, such as being anti-cancer, anti-viral, and anti-inflammatory. However, little is known about its effect on milk synthesis during lactation. Therefore, we selected different concentrations (5 mM, 10 mM, and 20 mM) of lithium chloride (LiCl) and assessed the effect of LiCl on bovine mammary epithelial (MAC-T) cells that underwent 4 days of differentiation induction. Moreover, we analyzed the effect of LiCl on the expression of genes related to milk fat and milk protein synthesis. Herein, LiCl (5–20 mM) significantly increased the expression of β-casein, promoted mRNA expression and phosphorylated protein expression of the signal transduction molecule and activator of transcription 5β (STAT5-β), and inhibited mRNA and protein expression of suppressor of cytokine signaling 2 (SOCS2). In contrast, 5 and 10 mM LiCl significantly inhibited expression of SOCS3. LiCl at concentration of 5–20 mM enhanced phosphorylation level of mTOR protein; at 10 mM and 20 mM, LiCl significantly promoted expression and phosphorylation of downstream ribosomal protein S6 kinase beta-1 (S6K1) protein. Considering milk fat synthesis, mRNA expression of acetyl CoA carboxylase (ACC) and lipoprotein lipase (LPL) genes was considerably increased in the presence of LiCl (5–20 mM). Additionally, increased protein expression levels of stearoyl-CoA desaturase (SCD), peroxisome proliferator–activated receptor-γ (PPARγ), and sterol regulatory element-binding protein 1 (SREBP1) were observed at all LiCl concentrations tested. Subsequently, LiCl (5–20 mM) significantly promoted protein expression and phosphorylation of β-catenin, while 10 mM and 20 mM of LiCl significantly promoted protein expression of hypoxia-inducible factor-1α (HIF-1α). Collectively, it has been shown that 10 mM LiCl can effectively activate HIF-1α, β-catenin, and β-catenin downstream signaling pathways. Conversely, at 10 mM, LiCl inhibited SOCS2 and SOCS3 protein expression through JAK2/STAT5, mTOR, and SREBP1 signaling pathways, improving synthesis of milk protein and fat. Therefore, LiCl can be used as a potential nutrient to regulate milk synthesis in dairy cows.








Similar content being viewed by others
Data Availability
Data are available for consultation upon request to the corresponding author.
References
Shahzad B, Mughal MN, Tanveer M, Gupta D, Abbas G (2017) Is lithium biologically an important or toxic element to living organisms? An overview. Environ Sci Pollut Res 24(1):103–115. https://doi.org/10.1007/s11356-016-7898-0
Nguyen LD, Fischer TT, Ehrlich BE (2021) Pharmacological rescue of cognitive function in a mouse model of chemobrain. Mol Neurodegener 16(1):41. https://doi.org/10.1186/s13024-021-00463-2
Anmella G, Fico G, Lotfaliany M, Hidalgo-Mazzei D, Soto-Angona O, Gimenez-Palomo A, Amoretti S, Murru A, Radua J, Solanes A, Pacchiarotti I, Verdolini N, Cowdery S, Dodd S, Williams LJ, Mohebbi M, Carvalho AF, Kessing LV, Vieta E, Berk M (2021) Risk of cancer in bipolar disorder and the potential role of lithium: international collaborative systematic review and meta-analyses. Neurosci Biobehav Rev 126:529–541. https://doi.org/10.1016/j.neubiorev.2021.03.034
Lee JH, Kim SW, Kim JH, Kim HJ, Um J, Jung DW, Williams DR (2021) Lithium chloride protects against sepsis-induced skeletal muscle atrophy and cancer cachexia. Cells 10(5):1017. https://doi.org/10.3390/cells10051017
Wen J, Sawmiller D, Wheeldon B, Tan J (2019) A review for lithium: pharmacokinetics, drug design, and toxicity. CNS Neurol Disord Drug Targets 18(10):769–778. https://doi.org/10.2174/1871527318666191114095249
Schrauzer GN (2002) Lithium: occurrence, dietary intakes, nutritional essentiality. J Am Coll Nutr 21(1):14–21. https://doi.org/10.1080/07315724.2002.10719188
Manuelian CL, Albanell E, Rovai M, Caja G, Guitart R (2015) Kinetics of lithium as a lithium chloride dose suitable for conditioned taste aversion in lactating goats and dry sheep. J Anim Sci 93(2):562–569. https://doi.org/10.2527/jas.2014-8223
Ralphs MH (1999) Lithium residue in milk from doses used to condition taste aversions and effects on nursing calves. Appl Anim Behav Sci 61(4):285–293. https://doi.org/10.1016/S0168-1591(98)00199-3
Alexander CM (2021) Wnt signaling and mammary stem cells. Vitam Horm 116:21–50. https://doi.org/10.1016/bs.vh.2021.02.001
Szyk-Warszynska L, Raszka K, Warszynski P (2019) Interactions of casein and polypeptides in multilayer films studied by FTIR and molecular dynamics. Polymers (Basel) 11(5):920. https://doi.org/10.3390/polym11050920
Greenberg R, Groves ML, Dower HJ (1984) Human beta-casein. Amino acid sequence and identification of phosphorylation sites. J Biol Chem 259(8):5132–5138
Jiang Q, He L, Hou Y, Chen J, Duan Y, Deng D, Wu G, Yin Y, Yao K (2016) Alpha-ketoglutarate enhances milk protein synthesis by porcine mammary epithelial cells. Amino Acids 48(9):2179–2188. https://doi.org/10.1007/s00726-016-2249-5
Yang L, Yang Q, Li F, Yi W, Liu F, Wang S, Jiang Q (2020) Effects of dietary supplementation of lauric acid on lactation function, mammary gland development, and serum lipid metabolites in lactating mice. Animals (Basel) 10(3):529. https://doi.org/10.3390/ani10030529
Zhong W, Shen J, Liao X, Liu X, Zhang J, Zhou C, Jin Y (2020) Camellia (Camellia oleifera Abel.) seed oil promotes milk fat and protein synthesis-related gene expression in bovine mammary epithelial cells. Food Sci Nutr 8(1):419–427. https://doi.org/10.1002/fsn3.1326
Liao XD, Zhou CH, Zhang J, Shen JL, Wang YJ, Jin YC, Li SL (2020) Effect of all-trans retinoic acid on casein and fatty acid synthesis in MAC-T cells. Asian-Australas J Anim Sci 33(6):1012–1022. https://doi.org/10.5713/ajas.19.0315
Yang JX, Wang CH, Xu QB, Zhao FQ, Liu JX, Liu HY (2015) Methionyl-methionine promotes alpha-s1 casein synthesis in bovine mammary gland explants by enhancing intracellular substrate availability and activating JAK2-STAT5 and mTOR-mediated signaling pathways. J Nutr 145(8):1748–1753. https://doi.org/10.3945/jn.114.208330
Ricoult SJH, Yecies JL, Ben-Sahra I, Manning BD (2016) Oncogenic PI3K and K-Ras stimulate de novo lipid synthesis through mTORC1 and SREBP. Oncogene 35(10):1250–1260. https://doi.org/10.1038/onc.2015.179
Fan Y, Yan LT, Yao Z, Xiong GY (2021) Biochanin A regulates cholesterol metabolism further delays the progression of nonalcoholic fatty liver disease. Diabetes Metab Syndr Obes 14:3161–3172. https://doi.org/10.2147/DMSO.S315471
Li N, Zhao F, Wei CJ, Liang MY, Zhang N, Wang CM, Li QZ, Gao XJ (2014) Function of SREBP1 in the milk fat synthesis of dairy cow mammary epithelial cells. Int J Mol Sci 15(9):16998–17013. https://doi.org/10.3390/ijms150916998
Yang PP, Li CS, Kou YY, Jiang YJ, Li DF, Liu SS, Lu YP, Hasegawa T, Li MQ (2021) Notum suppresses the osteogenic differentiation of periodontal ligament stem cells through the Wnt/Beta catenin signaling pathway. Arch Oral Biol 130:105211. https://doi.org/10.1016/j.archoralbio.2021.105211
Rattanawarawipa P, Pavasant P, Osathanon T, Sukarawan W (2016) Effect of lithium chloride on cell proliferation and osteogenic differentiation in stem cells from human exfoliated deciduous teeth. Tissue Cell 48:425–431. https://doi.org/10.1016/j.tice.2016.08.005
Heo JS, Lee SY, Lee JC (2010) Wnt/beta-catenin signaling enhances osteoblastogenic differentiation from human periodontal ligament fibroblasts. Mol Cells 30:449–454. https://doi.org/10.1007/s10059-010-0139-3
Zhang Q, Zhang Q, Li H, Zhao X, Zhang H (2021) LiCl induces apoptosis via CHOP/NOXA/Mcl-1 axis in human choroidal melanoma cells. Cancer Cell Int 21:96. https://doi.org/10.1186/s12935-021-01778-2
Chi YY, Shen JL, Zhang J, Shan AS, Niu SL, Zhou CH, Lee HG, Jin YC (2016) Lithium chloride’s inhibition of 3T3-L1 cell differentiation by regulating the Wnt/beta-catenin pathway and enhancing villin 2 expression. Food Sci Biotechnol 25:1147–1153. https://doi.org/10.1007/s10068-016-0183-7
Rius AG, Appuhamy JA, Cyriac J, Kirovski D, Becvar O, Escobar J, McGilliard ML, Bequette BJ, Akers RM, Hanigan MD (2010) Regulation of protein synthesis in mammary glands of lactating dairy cows by starch and amino acids. J Dairy Sci 93(7):3114–3127. https://doi.org/10.3168/jds.2009-2743
Mapes J, Li Q, Kannan A, Anandan L, Laws M, Lydon JP, Bagchi IC, Bagchi MK (2017) CUZD1 is a critical mediator of the JAK/STAT5 signaling pathway that controls mammary gland development during pregnancy. Plos Genet 13(3):e1006654. https://doi.org/10.1371/journal.pgen.1006654
Nan X, Bu D, Li X, Wang J, Wei H, Hu H, Zhou L, Loor JJ (2014) Ratio of lysine to methionine alters expression of genes involved in milk protein transcription and translation and mTOR phosphorylation in bovine mammary cells. Physiol Genomics 46(7):268–275. https://doi.org/10.1152/physiolgenomics.00119.2013
Cianciulli A, Calvello R, Porro C, Trotta T, Panaro MA (2017) Understanding the role of SOCS signaling in neurodegenerative diseases: current and emerging concepts. Cytokine Growth F R 37:67–79. https://doi.org/10.1016/j.cytogfr.2017.07.005
Sun M, Tang C, Liu J, Jiang W, Yu H, Dong F, Huang C, Rixiati Y (2021) Comprehensive analysis of suppressor of cytokine signaling proteins in human breast Cancer. BMC Cancer 21(1):696. https://doi.org/10.1186/s12885-021-08434-y
Geng Z, Shan X, Lian S, Wang J, Wu R (2021) LPS-induced SOCS3 antagonizes the JAK2-STAT5 pathway and inhibits beta-casein synthesis in bovine mammary epithelial cells. Life Sci 278:119547. https://doi.org/10.1016/j.lfs.2021.119547
Kim WS, Kim MJ, Kim DO, Byun JE, Huy H, Song HY, Park YJ, Kim TD, Yoon SR, Choi EJ, Jung H, Choi I (2017) Suppressor of cytokine signaling 2 negatively regulates NK cell differentiation by inhibiting JAK2 activity. Sci Rep 7:46153. https://doi.org/10.1038/srep46153
Zhuo MQ, Pan YX, Wu K, Xu YH, Luo Z (2017) Characterization and mechanism of phosphoinositide 3-kinases (PI3Ks) members in insulin-induced changes of protein metabolism in yellow catfish Pelteobagrus fulvidraco. Gen Comp Endocrinol 247:34–45. https://doi.org/10.1016/j.ygcen.2017.04.002
Reed AS, Unger EK, Olofsson LE, Piper ML, Myers MG Jr, Xu AW (2010) Functional role of suppressor of cytokine signaling 3 upregulation in hypothalamic leptin resistance and long-term energy homeostasis. Diabetes 59(4):894–906. https://doi.org/10.2337/db09-1024
Li S, Hosseini A, Danes M, Jacometo C, Liu J, Loor JJ (2016) Essential amino acid ratios and mTOR affect lipogenic gene networks and miRNA expression in bovine mammary epithelial cells. J Anim Sci Biotechnol 7:44. https://doi.org/10.1186/s40104-016-0104-x
Lee MS, Kim JS, Cho SM, Lee SO, Kim SH, Lee HJ (2015) Fermented Rhus verniciflua stokes extract exerts an antihepatic lipogenic effect in oleic-acid-induced HepG2 cells via upregulation of AMP-activated protein kinase. J Agric Food Chem 63(32):7270–7276. https://doi.org/10.1021/acs.jafc.5b01954
Bi Y, Yuan X, Zhu P, Chen Y, Chen G, Chang G (2020) A novel long noncoding RNA, ENSGALG00000021686, regulates the intracellular transport of fatty acids by targeting the FABP3 gene in chicken. Biochem Biophys Res Commun 528(4):706–712. https://doi.org/10.1016/j.bbrc.2020.05.133
Osorio JS, Lohakare J, Bionaz M (2016) Biosynthesis of milk fat, protein, and lactose: roles of transcriptional and posttranscriptional regulation. Physiol Genomics 48(4):231–256. https://doi.org/10.1152/physiolgenomics.00016.2015
Hao Z, Luo Y, Wang J, Hickford JGH, Zhou H, Hu J, Liu X, Li S, Shen J, Ke N, Liang W, Huang Z (2021) MicroRNA-432 inhibits milk fat synthesis by targeting SCD and LPL in ovine mammary epithelial cells. Food Funct 12(19):9432–9442. https://doi.org/10.1039/d1fo01260f
Rincon G, Islas-Trejo A, Castillo AR, Bauman DE, German BJ, Medrano JF (2012) Polymorphisms in genes in the SREBP1 signalling pathway and SCD are associated with milk fatty acid composition in Holstein cattle. J Dairy Res 79(1):66–75. https://doi.org/10.1017/S002202991100080X
Meng FG, Zhang XN, Liu SX, Wang YR, Zeng T (2020) Roles of peroxisome proliferator-activated receptor alpha in the pathogenesis of ethanol-induced liver disease. Chem Biol Interact 327:109176. https://doi.org/10.1016/j.cbi.2020.109176
Li XZ, Jiang H, Liu YQ, Tang JW, Shi JS, Yu XJ, Wang X, Du L, Lu Q, Li CL, Liu YW, Yin XX (2021) Sarsasapogenin restores podocyte autophagy in diabetic nephropathy by targeting GSK3beta signaling pathway. Biochem Pharmaco 192:114675. https://doi.org/10.1016/j.bcp.2021.114675
Sharma M, Chuang WW, Sun Z (2002) Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3beta inhibition and nuclear beta-catenin accumulation. J Biol Chem 277(34):30935–30941. https://doi.org/10.1074/jbc.M201919200
Perugorria MJ, Olaizola P, Labiano I, Esparza-Baquer A, Marzioni M, Marin JJG, Bujanda L, Banales JM (2019) Wnt-beta-catenin signalling in liver development, health and disease. Nat Rev Gastro Hepat 16(2):121–136. https://doi.org/10.1038/s41575-018-0075-9
Chen J, Liu W, Lee KF, Liu K, Wong BPC, Yeung Shu-Biu W (2021) Overexpression of Lin28a induces a primary ovarian insufficiency phenotype via facilitation of primordial follicle activation in mice. Mol Cell Endocrinol 539:111460. https://doi.org/10.1016/j.mce.2021.111460
Cai CG, Yu QC, Jiang WM, Liu W, Song WQ, Zhang YuH, L, Yang Y, Zeng YA, (2014) R-spondin1 is a novel hormone mediator for mammary stem cell self-renewal. Gene Dev 28(20):2205–2218. https://doi.org/10.1101/gad.245142.114
Bagchi DP, Nishii A, Li ZR, DelProposto JB, Corsa CA, Mori H, Bagchi DP, Nishii A, Li ZR, DelProposto JB, Corsa CA, Mori H, Hardij J, Learman BS, Lumeng CN, MacDougald OA (2020) Wnt/beta-catenin signaling regulates adipose tissue lipogenesis and adipocyte-specific loss is rigorously defended by neighboring stromal-vascular cells. Mol Metab 42:101078. https://doi.org/10.1016/j.molmet.2020.101078
Wang J, Ling R, Zhou Y, Gao X, Yang Y, Mao C, Chen D (2020) SREBP1 silencing inhibits the proliferation and motility of human esophageal squamous carcinoma cells via the Wnt/beta-catenin signaling pathway. Oncol Lett 20(3):2855–2869. https://doi.org/10.3892/ol.2020.11853
Tong JL, Wang LL, Ling XF, Wang MX, Ca W, Liu YY (2019) MiR-875 can regulate the proliferation and apoptosis of non-small cell lung cancer cells via targeting SOCS2. Eur Rev Med Pharmacol Sci 23(12):5235–5241. https://doi.org/10.26355/eurrev_201906_18189
Gong R, Xi Y, Jin X, Xu H, Feng J, Hu Q, Xia Z (2021) Effects of the decrease of β-catenin expression on human vaginal fibroblasts of women with pelvic organ prolapse. J Obstet Gynaecol Res 47(11):4041–4022. https://doi.org/10.1111/jog.14946
Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA (2000) Inhibition of adipogenesis by Wnt signaling. Science 289(5481):950953. https://doi.org/10.1126/science.289.5481.950
ShaoY ZFQ (2014) Emerging evidence of the physiological role of hypoxia in mammary development and lactation. J Anim Sci Biotechnol 5(1):9. https://doi.org/10.1186/2049-1891-5-9
Badowska-Kozakiewicz AM, Sobol M, Patera J (2017) Expression of multidrug resistance protein P-glycoprotein in correlation with markers of hypoxia (HIF-1 alpha, EPO, EPO-R) in invasive breast cancer with metastasis to lymph nodes. Arch Med Sci 13(6):1303–1314. https://doi.org/10.5114/aoms.2016.62723
Cokic VP, Bhattacharya B, Beleslin-Cokic BB, Noguchi CT, Puri RK, Schechter AN (2012) JAK-STAT and AKT pathway-coupled genes in erythroid progenitor cells through ontogeny. J Transl Med 10:116. https://doi.org/10.1186/1479-5876-10-116
Choi K, Jin M, Zouboulis CC, Lee Y (2021) Increased lipid accumulation under hypoxia in SZ95 human sebocytes. Dermatology 237(1):131–141. https://doi.org/10.1159/000505537
Lee HJ, Jung YH, Choi GE, Ko SH, Lee SJ, Lee SH, Han HJ (2017) BNIP3 induction by hypoxia stimulates FASN-dependent free fatty acid production enhancing therapeutic potential of umbilical cord blood-derived human mesenchymal stem cells. Redox Biol 13:426–443. https://doi.org/10.1016/j.redox.2017.07.004
Penna F, Busquets S, Toledo M, Pin F, Massa D, Lopez-Soriano FJ, Costelli P, Argiles JM (2013) Erythropoietin administration partially prevents adipose tissue loss in experimental cancer cachexia models. J Lipid Res 54(11):3045–3051. https://doi.org/10.1194/jlr.M038406
Qi C, Zhang J, Chen X, Wan J, Wang J, Zhang P, Liu Y (2017) Hypoxia stimulates neural stem cell proliferation by increasing HIF1alpha expression and activating Wnt/beta-catenin signaling. Cell Mol Biol (Noisy-le-grand) 63(7):12–19. https://doi.org/10.14715/cmb/2017.63.7.2
Vallée A, Guillevin R, Vallée JN (2018) Vasculogenesis and angiogenesis initiation under normoxic conditions through Wnt/β-catenin pathway in gliomas. Rev Neurosci 29(1):71–91. https://doi.org/10.1515/revneuro-2017-0032
Jiang YG, Luo Y, He DL, Li X, Zhang LL, Peng T, Li MC, Lin YH (2007) Role of Wnt/beta-catenin signaling pathway in epithelial-mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1alpha. Int J Urol 14:1034–1039. https://doi.org/10.1111/j.1442-2042.2007.01866.x
Zhang Q, Bai X, Chen W, Ma T, Hu Q, Liang C, Xie S, Chen C, Hu L, Xu S, Liang T (2013) Wnt/beta-catenin signaling enhances hypoxia-induced epithelial-mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1alpha signaling. Carcinogenesis 34:962–973. https://doi.org/10.1093/carcin/bgt027
Vallee A, Levy BL, Blacher J (2018) Interplay between the renin-angiotensin system, the canonical WNT/beta-catenin pathway and PPARgamma in hypertension. Curr Hypertens Rep 20:62. https://doi.org/10.1007/s11906-018-0860-4
Patten DA, Lafleur VN, Robitaille GA, Chan DA, Giaccia AJ, Richard DE (2010) Hypoxia-inducible factor-1 activation in nonhypoxic conditions: the essential role of mitochondrial-derived reactive oxygen species. Mol Biol Cell 21:3247–3257. https://doi.org/10.1091/mbc.E10-01-0025
Kurlak LO, Williams PJ, Bulmer JN, Broughton Pipkin F, Mistry HD (2015) Placental expression of adenosine A(2A) receptor and hypoxia inducible factor-1 alpha in early pregnancy, term and pre-eclamptic pregnancies: interactions with placental renin-angiotensin system. Placenta 36:611–613. https://doi.org/10.1016/j.placenta.2015.02.011
Seagroves TN, Hadsell D, McManaman J, Palmer C, Liao D, McNulty W, Welm B, Wagner KU, Neville M, Johnson RS (2003) HIF1 alpha a is a critical regulator of secretory differentiation and activation, but not vascular expansion, in the mouse mammary gland. Development 130:1713–1724. https://doi.org/10.1242/dev.00403
Cassavaugh J, Lounsbury KM (2011) Hypoxia-mediated biological control. J Cell Biochem 112:735–744. https://doi.org/10.1002/jcb.22956
Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL (2003) Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res 93:1074–1081. https://doi.org/10.1161/01.RES.0000102937.50486.1B
Rankin EB, Giaccia AJ (2008) The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ 15:678–685. https://doi.org/10.1038/cdd.2008.21
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16:4604–4613. https://doi.org/10.1128/MCB.16.9.4604
Cai J, Wang DM, Liu JX (2018) Regulation of fluid flow through the mammary gland of dairy cows and its effect on milk production: a systematic review. J Sci Food Agr 98:1261–1270. https://doi.org/10.1002/jsfa.8605
Morimoto H, Yamamoto T, Miyazaki T, Ogonuki N, Ogura A, Tanaka T, Kanatsu-Shinohara M, Yabe-Nishimura C, Zhang H, Pommier Y, Trumpp A, Shinohara T (2021) An interplay of NOX1-derived ROS and oxygen determines the spermatogonial stem cell self-renewal efficiency under hypoxia. Genes Dev 35:250–260. https://doi.org/10.1101/gad.339903.120
Agani FH, Pichiule P, Chavez JC, LaManna JC (2000) The role of mitochondria in the regulation of hypoxia-inducible factor 1 expression during hypoxia. J Biol Chem 275:35863–35867. https://doi.org/10.1074/jbc.M005643200
Zhao RZ, Jiang S, Zhang L, Yu ZB (2019) Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int J Mol Med 44:3–15. https://doi.org/10.3892/ijmm.2019.4188
Kannan N, Nguyen LV, Makarem M, Dong Y, Shih K, Eirew P, Raouf A, Emerman JT, Eaves CJ (2014) Glutathione-dependent and -independent oxidative stress-control mechanisms distinguish normal human mammary epithelial cell subsets. Proc Natl Acad Sci U S A 111:7789–7794. https://doi.org/10.1073/pnas.1403813111
Sordillo LM, Pighetti GM, Davis MR (1995) Enhanced production of bovine tumor necrosis factor-alpha during the periparturient period. Vet Immunol Immunopathol 49:263–270. https://doi.org/10.1016/0165-2427(95)05465-0
Liu XL, Shen JL, Zong JX, Liu JY, Jin YC (2021) Beta-sitosterol promotes milk protein and fat syntheses-related genes in bovine mammary epithelial cells. Animals (Basel) 11(11):3238. https://doi.org/10.3390/ani11113238
Hadaya O, Bransi-Nicola R, Shalev Y, Azaizeh H, Roth Z, Muklada H, Deutch T, Landau SY, Argov-Argaman N (2020) Pistacia lentiscus extract enhances mammary epithelial cells' productivity by modulating their oxidative status. Sci Rep 10(1):20985. https://doi.org/10.1038/s41598-020-78065
Chen W, Wei W, Yu L, Zhang X, Huang F, Zheng Q, Wang L, Cai C (2021) Baicalin promotes mammary gland development via steroid-like activities. Front Cell Dev Biol 9:682469. https://doi.org/10.3389/fcell.2021.682469
Hadaya O, Landau SY, Glasser T, Muklada H, Deutch T, Shemesh M, Argov-Argaman N (2020) Producing pasture-like milk from goats in confinement. Livest Sci 236:104056. https://doi.org/10.1016/j.livsci.2020.10405
Manuelian CL, Albanell E, Rovai M, Caja G (2016) How to create conditioned taste aversion for grazing ground covers in woody crops with small ruminants. J Vis Exp (110):53887. https://doi.org/10.3791/53887
Acknowledgements
We gratefully acknowledge Professor Hong-Gu Lee (Department of Animal Science and Technology, Sanghuh College of Life Sciences, Konkuk University, Seoul 05029, Korea) for generously providing MAC-T cells for cell culture assays.
Funding
This study was funded by the Jilin Provincial Department of Education (grant number JJKH20201022KJ) and the National Natural Science Foundation of China (grant number 31301996).
Author information
Authors and Affiliations
Contributions
Y. Jin, J. Shen and J. Zhang: conceptualization; J. Zong: methodology; J. Zong, X. Liu and J. Liu: validation; X. Liu, J. Liu and Y. Fan: investigation; J. Shen, J. Zhang and C. Zhou: resources; J. Zong: data curation; J. Zong: writing—original draft preparation; Y. Jin: writing—review and editing; J. Zong: visualization; Y. Jin and J. Shen: supervision; Y. Jin: project administration; Y. Jin: funding acquisition. All authors have read and agreed to the current version of the manuscript.
Corresponding author
Ethics declarations
Disclaimer
The funders had no role in the design of the study; in data collection, analysis, or interpretation; in manuscript writing; or in publishing decision criteria.
Ethical Approval
This study did not involve any human or animal testing.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zong, J., Shen, J., Liu, X. et al. Lithium Chloride Promotes Milk Protein and Fat Synthesis in Bovine Mammary Epithelial Cells via HIF-1α and β-Catenin Signaling Pathways. Biol Trace Elem Res 201, 180–195 (2023). https://doi.org/10.1007/s12011-022-03131-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03131-8