Abstract
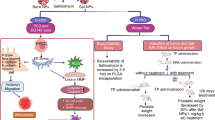
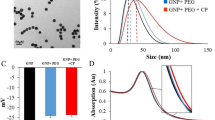
Nanotechnology is a possible solution to the drawbacks of cancer therapy because it decreases the clinical side effects of chemotherapeutic drugs and increases their clinical activity. Thus, this work compared the in vitro cytotoxic activity and in vivo side effects of cisplatin (CP) with those of CP-loaded green silver nanoparticles (CP-AgNPs). The cytotoxic activity of CP, green AgNPs, and CP-AgNPs against PC-3, a human prostate cancer cell line, was assessed using MTT assay. CP-AgNPs had a superior cytotoxic effect on PC-3 cells with a 50% inhibition of viability (IC50) of 27.05 μg/mL, followed by CP with an IC50 of 57.64 μg/mL and AgNPs with an IC50 125.4 μg/mL. To evaluate in vivo side effects, 40 male adult Wistar rats were assigned into four groups and intraperitoneally injected with normal saline (control), CP (2.5 mg/kg body weight), green AgNPs (0.1 mL/kg body weight), and CP-AgNPs (2.5 mg/kg body weight). Intraperitoneal CP injection caused a substantial reduction in erythrocyte and leukocyte counts and hemoglobin concentration and a marked increase in urea and creatinine levels and disturbed the renal oxidant/antioxidant status. Furthermore, it caused noticeable structural alterations and significant upregulation of renal Bax and caspase-3 mRNA along with a significant downregulation of B-cell lymphoma 2 mRNA expressions. The loading of CP on green AgNPs significantly relieved the CP-induced pathological alterations and considerably enhanced its therapeutic effectiveness on PC-3 cells. These outcomes reflect the possible use of CP-AgNPs as a more efficient and safer anticancer agent than free CP.



Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request
References
Dasari S, Tchounwou PB (2014) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740:364–378. https://doi.org/10.1016/j.ejphar.2014.07.025
Fuertes MA, Alonso C, Pérez JM (2003) Biochemical modulation of cisplatin mechanisms of action: enhancement of antitumor activity and circumvention of drug resistance. Chem Rev 103(3):645–662. https://doi.org/10.1021/cr020010d
Casares C, Ramírez-Camacho R, Trinidad A, Roldán A, Jorge E, García-Berrocal JR (2012) Reactive oxygen species in apoptosis induced by cisplatin: review of physiopathological mechanisms in animal models. Eur Arch Otorhinolaryngol 269(12):2455–2459
Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G (2012) Molecular mechanisms of cisplatin resistance. Oncogene 31(15):1869–1883. https://doi.org/10.1038/onc.2011.384
Kelland L (2007) The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 7(8):573–584
Duan X, He C, Kron SJ, Lin W (2016) Nanoparticle formulations of cisplatin for cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol 8(5):776–791. https://doi.org/10.1002/wnan.1390
Alsaab HO (2018) Tumor multicomponent targeting polymer-lipid hybrid nanoparticles to overcome drug resistance in renal cell carcinoma. Wayne State University
Zhang X-F, Liu Z-G, Shen W, Gurunathan S (2016) Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci 17(9):1534. https://doi.org/10.3390/ijms17091534
Osama E, El-Sheikh SM, Khairy MH, Galal AA (2020) Nanoparticles and their potential applications in veterinary medicine. J Adv Vet Res 10(4):268–273
Liu J, Abshire C, Carry C, Sholl AB, Mandava SH, Datta A, Ranjan M, Callaghan C, Peralta DV, Williams KS, Lai WR, Abdel-Mageed AB, Tarr M, Lee BR (2017) Nanotechnology combined therapy: tyrosine kinase-bound gold nanorod and laser thermal ablation produce a synergistic higher treatment response of renal cell carcinoma in a murine model. BJU Int 119(2):342–348. https://doi.org/10.1111/bju.13590
Sevilla P, De Llanos R, Domingo C, Sánchez-Cortés S, García-Ramos J (2010) SERS+MEF of the anti-tumoral drug emodin adsorbed on silver nanoparticles, vol 7577. SPIE, SPIE BiOS
Ramezani T, Nabiuni M, Baharara J, Parivar K, Namvar F (2019) Sensitization of resistance ovarian cancer cells to cisplatin by biogenic synthesized silver nanoparticles through p53 activation. Iran J Pharm Res: IJPR 18(1):222–231
El-Sheikh SM, Khairy MH, Osama E, Metwally MM, Galal AA (2020) Nanotechnology improves the therapeutic efficacy of gemcitabine against a human hepatocellular carcinoma cell line and minimizes its in vivo side effects. Naunyn Schmiedeberg's Arch Pharmacol 1–13. https://doi.org/10.1007/s00210-020-02004-y
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7(2):27–31. https://doi.org/10.4103/0976-0105.177703
Devi LS, Joshi SR (2012) Antimicrobial and synergistic effects of silver nanoparticles synthesized using soil fungi of high altitudes of Eastern Himalaya. Mycobiology 40(1):27–34. https://doi.org/10.5941/myco.2012.40.1.027
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1):55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Yang Y, Du X, Wang Q, Liu J, Zhang E, Sai L, Peng C, Lavin MF, Yeo AJ, Yang X, Shao H, Du Z (2019) Mechanism of cell death induced by silica nanoparticles in hepatocyte cells is by apoptosis. Int J Mol Med 44(3):903–912. https://doi.org/10.3892/ijmm.2019.4265
El-Sonbaty SM (2013) Fungus-mediated synthesis of silver nanoparticles and evaluation of antitumor activity. Cancer Nanotechnol 4(4):73–79. https://doi.org/10.1007/s12645-013-0038-3
Kaplan A (1969) The determination of urea, ammonia, and urease. In: Methods of biochemical analysis. pp 311–324. https://doi.org/10.1002/9780470110355.ch7
Toora BD, Rajagopal G (2002) Measurement of creatinine by Jaffe’s reaction—determination of concentration of sodium hydroxide required for maximum color development in standard, urine and protein free filtrate of serum. Indian J Exp Biol 40(3):352–354
Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V (2001) Method for the measurement of antioxidant activity in human fluids. J Clin Pathol 54(5):356–361. https://doi.org/10.1136/jcp.54.5.356
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods (San Diego, Calif) 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Suvarna SK, Layton C, Bancroft JD (2018) Bancroft’s theory and practice of histological techniques, 8th edn. Churchill Livingstone Elsevier, Oxford
Galal AAA, Ramadan RA, Metwally MMM, El-Sheikh SMA (2019) Protective effect of N-acetylcysteine on fenitrothion-induced toxicity: the antioxidant status and metabolizing enzymes expression in rats. Ecotoxicol Environ Saf 171:502–510. https://doi.org/10.1016/j.ecoenv.2019.01.004
Kang X, Xiao H-H, Song H-Q, Jing X-B, Yan L-S, Qi R-G (2015) Advances in drug delivery system for platinum agents based combination therapy. Cancer Biol Med 12(4):362–374. https://doi.org/10.7497/j.issn.2095-3941.2015.0063
Kashani S, Kilbas PO, Yerlikaya PO, Gurkan AC, Arisan ED (2020) Cisplatin and paclitaxel modulated the cell survival potential of prostate cancer cells. Multidiscip Dig Publ Instit Proc 40(1):42
Ghaferi M, Amari S, Mohrir BV, Raza A, Shahmabadi HE, Alavi SE (2020) Preparation, characterization, and evaluation of cisplatin-loaded polybutylcyanoacrylate nanoparticles with improved in vitro and in vivo anticancer activities. Pharmaceuticals (Basel, Switzerland) 13(3). https://doi.org/10.3390/ph13030044
Ghaferi M, Asadollahzadeh MJ, Akbarzadeh A, Ebrahimi Shahmabadi H, Alavi SE (2020) Enhanced efficacy of PEGylated liposomal cisplatin: in vitro and in vivo evaluation. Int J Mol Sci 21(2):559. https://doi.org/10.3390/ijms21020559
Zaied FA, Elballat SE (2019) Reduction in the formation of micronucleated polychromatic erythrocytes induced by cisplatin in bone marrow cells of rats by using antioxidants. Egypt Acad J Biol Sci C, Physiol Mol Biol 11(2):47–55. https://doi.org/10.21608/eajbsc.2019.33962
Karale S, Kamath JV (2017) Effect of daidzein on cisplatin-induced hematotoxicity and hepatotoxicity in experimental rats. Indian J Pharm 49(1):49–54. https://doi.org/10.4103/0253-7613.201022
Pabla N, Dong Z (2008) Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73(9):994–1007
Bolisetty S, Traylor A, Joseph R, Zarjou A, Agarwal A (2016) Proximal tubule-targeted heme oxygenase-1 in cisplatin-induced acute kidney injury. Am J Physiol Ren Physiol 310(5):F385–F394. https://doi.org/10.1152/ajprenal.00335.2015
Bazmandegan G, Amirteimoury M, Kaeidi A, Shamsizadeh A, Khademalhosseini M, Nematollahi MH, Hassanipour M, Fatemi I (2019) Sumatriptan ameliorates renal injury induced by cisplatin in mice. Iran J Basic Med Sci 22(5):563–567. https://doi.org/10.22038/ijbms.2019.33620.8020
Abdel-Daim MM, Mahmoud OM, Al Badawi MH, Alghamdi J, Alkahtani S, Salem NA (2020) Protective effects of Citrus limonia oil against cisplatin-induced nephrotoxicity. Environ Sci Pollut Res 27(33):41540–41550. https://doi.org/10.1007/s11356-020-10066-x
Kannan K, Jain SK (2000) Oxidative stress and apoptosis. Pathophysiology: Off J Int Soc Pathophysiol 7(3):153–163. https://doi.org/10.1016/s0928-4680(00)00053-5
Tsujimoto Y (1998) Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells: Devoted Mol Cell Mech 3(11):697–707. https://doi.org/10.1046/j.1365-2443.1998.00223.x
Volarevic V, Djokovic B, Jankovic MG, Harrell CR, Fellabaum C, Djonov V, Arsenijevic N (2019) Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J Biomed Sci 26(1):25. https://doi.org/10.1186/s12929-019-0518-9
Ni J, Hou X, Wang X, Shi Y, Xu L, Zheng X, Liu N, Qiu A, Zhuang S (2019) 3-Deazaneplanocin A protects against cisplatin-induced renal tubular cell apoptosis and acute kidney injury by restoration of E-cadherin expression. Cell Death Dis 10(5):355. https://doi.org/10.1038/s41419-019-1589-y
Zhang Z, Xin G, Zhou G, Li Q, Veeraraghavan VP, Krishna Mohan S, Wang D, Liu F (2019) Green synthesis of silver nanoparticles from Alpinia officinarum mitigates cisplatin-induced nephrotoxicity via down-regulating apoptotic pathway in rats. Artif Cells Nanomed Biotechnol 47(1):3212–3221. https://doi.org/10.1080/21691401.2019.1645158
Gad A, Kydd J, Piel B, Rai P (2016) Targeting cancer using polymeric nanoparticle mediated combination chemotherapy. Int J Nanomed Nanosurg 2(3). https://doi.org/10.16966/2470-3206.116
Parhi P, Mohanty C, Sahoo SK (2012) Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discov Today 17(17-18):1044–1052. https://doi.org/10.1016/j.drudis.2012.05.010
Poon C, He C, Liu D, Lu K, Lin W (2015) Self-assembled nanoscale coordination polymers carrying oxaliplatin and gemcitabine for synergistic combination therapy of pancreatic cancer. J Control Release 201:90–99. https://doi.org/10.1016/j.jconrel.2015.01.026
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 4093 kb)
Rights and permissions
About this article
Cite this article
El-Sheikh, S.M.A., Edrees, N., EL-Sayed, H. et al. Could Cisplatin Loading on Biosynthesized Silver Nanoparticles Improve Its Therapeutic Efficacy on Human Prostate Cancer Cell Line and Reduce Its In Vivo Nephrotoxic Effects?. Biol Trace Elem Res 200, 582–590 (2022). https://doi.org/10.1007/s12011-021-02677-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02677-3