Abstract
Intradialytic hypertension (iHTN) has been related with an increased risk of mortality, with imbalances in trace elements being frequent in maintenance hemodialysis (MHD) patients. The aim of this study was to analyze the relationships between the levels of blood trace elements and iHTN in MHD patients. A total of 144 MHD patients were enrolled in September, 2019 (66 females; 5616 hemodialysis treatments), with a mean age of 64.33 ± 13.39 years and median vintage of 33.50 (16.25–57.50) months. Patients exhibited an average peridialytic systolic blood pressure (SBP) change of − 4.18 ± 20.22 mm Hg in the next 3 months. Thirty-four (23.6%) patients had persistent iHTN (piHTN). These patients were characterized by older age, higher rate of hypozincemia, and modified Charlson comorbidity score, whereas lower blood zinc and hemoglobin, at the time of their recruitment. No significant difference in the levels of other blood trace elements was observed between groups. A general linear mixed (GLM) model showed that with every mg/L point lower mean blood zinc at baseline, the peridialytic SBP change was increased by 4.524 mm Hg (P < 0.001). Binary logistic model in modulate of the GLM model revealed that the lower level of blood zinc was associated with piHTN (OR = 0.433, 95 % CI 0.295 to 0.637, P < 0.001). Multivariate analysis confirmed both above results. Our study indicated that lower blood zinc was independently associated with piHTN in patients undergoing MHD, but prospective studies with larger population are still needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although hemodialysis (HD) is known to promote a reduction in the levels of blood pressure (BP) in most hypertensive patients, because of the removal of fluids and salt [1], a minority of patients have been shown to regularly exhibit a paradoxical increase either toward or at the end of HD [2]. This phenomenon has been termed intradialytic hypertension (iHTN). There is no consensus regarding its definition, but clinical studies have recently reported iHTN during HD as a rise in the systolic blood pressure (SBP) greater than 10 mm Hg above the level of predialysis SBP [3,4,5]. Results from cohort studies with a larger sample size showed that 8–13% of HD patients had iHTN [2, 5]. Compared with patients whose SBP were lower during a HD session, patients with iHTN were observed to have an increased risk for hospitalization and mortality [6]. The underlying mechanism of iHTN, which is likely multifactorial, remains to be determined. Studies have shown that older age, shorter dialysis vintage, increased cardiovascular comorbidities, lower body mass index, and lower amount of ultrafiltration during dialysis were the main clinical characteristics of HD patients with iHTN [6]. In addition, activation of the renin–angiotensin–aldosterone system [7], positive sodium balance [8], endothelial dysfunction [4, 9], low intradialytic arterial oxygen saturation [10], and excess extracellular fluid [3] were also considered to be related factors.
Imbalances in trace elements have been associated with the development of cardiovascular disease (CVD), including hypertension [11]. Both hypozincemia and zinc transporter protein abnormalities have been linked to atherosclerosis and microvascular disease [12]. Studies have reported a positive relationship between the levels of serum copper and acute or chronic heart failure [13]. Evidence has also been provided for the important functions of selenium and its selenoproteins in certain CVD, such as Keshan disease and myocardial infarct [14]. Manganese could potentially play a role in controlling blood pressure due to its antioxidative function [15], and significant negative associations between levels of urinary manganese and both systolic and diastolic blood pressure were demonstrated after adjusting for confounding factors [16]. The imbalance of trace elements has been reported to be very common in HD patients [17], and although iHTN belongs to the CVD category associated with the vascular function, rarely studies have focused on the associations between blood or serum trace elements and iHTN. Our study aimed to test the hypothesis that the blood levels of certain trace elements at baseline might be associated with iHTN in HD patients.
Materials and Methods
Study Population
Patients undergoing maintenance hemodialysis (MHD) were enrolled in this study in September 2019 at the HD center of the Guangzhou Red Cross Hospital, Jinan University. Inclusion criteria were as follows: (1) HD therapy for more than 3 months, (2) age ≥ 18 years, (3) treated with bicarbonate dialysate and polysulfone membranes, and (4) signed informed consent form. Exclusion criteria were as follows: (1) patients with an expected survival time of less than 3 months or expected to have kidney transplantation within 3 months; (2) patients who had a history of trauma, surgery, or serious infection within the last 3 months; (3) patients who had a confirmed diagnosis of malignancies; (4) patients who refused participating in the study; and (5) patients who were taking trace element supplements or drugs affecting the metabolism of trace elements. Clinical, biochemical, and blood trace elements data were collected following enrollment in the study. Drug usage and dosage were also recorded in the next 3 months. Patients were censored in the event of dialysis modality change, kidney transplantation, transfer to other centers, or recovery of kidney function.
Patients enrolled in the study were subjected to hemodialysis and hemodiafiltration treatments 3 times a week. The machines used were Braun Dialog +(B. Braun Co., Ltd., Melsungen, Germany). A REXEED-15L high-flux polysulfone membrane dialyzer (Asahi Kasei Corp., Tokyo, Japan) with a membrane area of 1.5 m2, a blood flow of 180–300 mL/min, a dialysate flow rate of 500 mL/min, and a dialysis time of 4 h, with. Ultra-pure water dialysate was used, and the iron concentrations of dialysate were as follows, respectively: Na+ 137.8 mmol/L, K+ 2.0 mmol/L, Ca2+ 1.5 mmol/L, Mg2+ 0.5 mmol/L, Cl– 108.7 mmol/L, CH3COO– 4.0 mmol/L, and HCO3– 31.1 mmol/L.
Measurement of Blood Pressure
The Omron HEM-7133 electronic sphygmomanometer (OMRON Corp., Kyoto, Japan) was used for the measurement of BP in the non-fistula side of the upper arm, and in the right side of the upper arm in patients with catheters. Predialytic BP (preHD BP) was measured after resting for 30 min, whereas postdialytic BP (postHD BP) was measured shortly after the end of dialysis. Accordingly, the peridialytic change in BP was calculated as follows: Peridialytic BP change = postHD BP − preHD BP. All patients were followed for 3 months, and their postHD BPs, postHD BPs, and peridialytic SBP changes were recorded in every HD treatment. Only patients with an average peridialytic SBP increase ≥ 10 mm Hg throughout the entire 3-month observation period were defined as patients with persistent iHTN (piHTN). Sphygmomanometers in our center were regularly calibrated by the manufacturer.
Demographic, clinical, and laboratorial data
Demographic, clinical, and laboratorial variables were collected following the enrollment of patients in the study.
Demographic variables were as follows: age; gender; predialytic body weight and height; primary kidney disease; smoking; interdialytic weight gain (IDWG); ultrafiltration volume. Drug usage in the next 3 months was also recorded: dosage of recombinant human erythropoietin (rHuEPO); usage of low calcium concentration dialysate (1.5 mmol/L); usage of angiotensin converting enzyme inhibitor (ACEI), angiotensin II receptor blocker (ARB), calcium channel blocker (CCB), β-receptor blocker, α-receptor blocker, and diuretics.
Venous blood samples were collected shortly before the HD session, and then were taken to the clinical laboratory department of our hospital for routine blood and biochemical tests to evaluate the following parameters: red blood cell (RBC), hemoglobin (Hb), hematocrit (Hct),serum creatinine (SCr), serum urea nitrogen (BUN), serum potassium ion (K+), serum sodium ion (Na+), serum magnesium ion (Mg2+), serum calcium ion (Ca2+), serum phosphorus (P), parathyroid hormone (PTH), and albumin (ALB). The weekly total Kt/V was measured using the methods described by Gotch et al. [18].
Blood Trace Element Test
Sample Preparation
Venous blood samples before dialysis treatment were collected at the time of the enrollment of patients in the study. After thorough mixing of the blood sample, 100 μL of it was taken to make a 1:19 dilution with diluents containing 0.1 % nitric acid (stock solution 65%, Sigma-Aldrich Co., Ltd., St. Louis, MO, USA) and 0.1% triton (stock solution X-100, 1.01 g/cm3, Sigma-Aldrich Co., Ltd., St. Louis, MO, USA) for further use.
Quantification of Trace Elements and Standard Curves
Levels of blood trace elements (zinc, manganese, copper, and selenium) were measured using an inductively coupled plasma mass spectrometer (ICP-MS, Agilent 7900, Agilent Technologies Inc., CA, USA). Instrument parameters were as follows: forward power 1550 W; carry gas (Argon) 1.0 L/min; aux gas (Argon) 1.0 L/min; plasma gas (Argon) 15.0 L/min; S/C temperature 2 °C; uptake speed 0.2 r/s; sample depth 6–8 mm; uptake time 30 s; acquisition time 0.1 s; repetition 2; cell entrance − 40 V; cell exit − 60 V; deflect 0 V; plate bias − 60 V; octP RF 180 V; octP bias − 18 V; QP bias − 15 V: gas flow (He) 4.0–5.0 mL/min. All selected trace elements were measured under optimum analytical conditions, as shown in Table 1. The tuning solution used for application (10.0 μg/L) contained lithium/cobalt/cerium/yttrium/thallium elements, while the internal standard solution used for application (20.0 μg/L) contained germanium/rhodium/indium/lutetium elements.
Modified Comorbidity Index
Modified Charlson comorbidity (CCI), which has been widely used in hemodialysis patients, was employed to assess the comorbidities of patients [19] at the time of their enrollment in the study.
Statistical Analysis
Descriptive statistics comprised mean ± standard deviation for continuous variables with normal distribution, median (25–75% interquartile range) for data with a skewed distribution, and percentages for categorical variables, respectively. Patients were stratified based on average peridialytic SBP changes recorded during the following 3-month observation period in the piHTN group (average peridialytic SBP change ≥ 10 mm Hg) and the non-piHTN group (average peridialytic SBP change ˂ 10 mm Hg). Differences between the 2 groups were evaluated using the Student’s t test or Mann–Whitney test or χ2 test, as appropriate. Both general linear mixed (GLM) and binary logistic regression models in the GLM module of SPSS were performed with univariate and multivariate analysis to determine the variables significantly affecting peridialytic SBP change and piHTN respectively. Repeated-measured data of blood pressure–related variables were applied into the GLM model. Confounding factors were selected based on their documented or hypothesized association with exposure and outcome. The SPSS version 24.0 for Windows (IBM Corp, Armonk, NY, USA) was used for statistical analysis. A P value < 0.05 was considered to be statistically significant.
Results
Characteristics of Study Population
A total of 144 MHD patients were included in the study in September, 2019. Patients had a mean age of 64.33 ± 13.39 years, median vintage of 33.50 (16.25–57.50) months, with 66 being females (45.8%). The primary cause of end-stage renal disease was diabetic nephropathy (n = 63, 43.8%), followed by hypertension (n = 25, 17.4%), and chronic glomerulonephritis (n = 16, 11.1%). Detailed characteristics of study population are provided in Table 2. After 3 months of follow-up, no patient had dropped out and all patients had completed 5616 HD treatments in total.
Peridialytic SBP Change and Levels of Trace Elements
At the time of their enrollment in the study, the mean preHD, postHD SBP, and peridialytic SBP change values of patients were 143.47 ± 18.79, 143.47 ± 18.79, and 1.85 ± 19.24 mm Hg, respectively. In the next 3 months, the mean peridialytic SBP change of all patients was shown to be − 4.18 ± 20.22 mm Hg. Figure 1 displays the frequency of iHTN. On average, all patients exhibited iHTN in 14.10 (5.13–32.69) of their treatments.
The mean level of blood zinc in these patients was 5.46 ± 1.38 mg/L, with a normal distribution. The median levels of blood copper, selenium, and manganese were 835.25 (752.38–962.15) μg/L, 103.15 (91.71–112.92) μg/L, and 13.55 (9.93–17.48) μg/L respectively; all with abnormal distributions. Following the recently published prospective study with a large population of MHD patients [20], all cutoffs of trace elements analyzed by ICP-MS in our study were according to those set by Cesbron et al. [21]. According to these cutoffs, a total of 30 patients were identified with hypocupremia, 2 with hypercupremia, 29 with hypozincemia, 18 with hyperzincemia, 10 with hypomanganesemia, 75 with hypermanganesemia, 17 with hyposelenemia, and 6 with hyperselenemia (Table 3).
Comparison between piHTN and Non-piHTN Patients
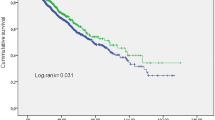
Thirty-four (23.6 %) patients were identified with having piHTN. A comparison between the 2 groups at the time of their enrollment in the study is displayed in Tables 2 and 3. Peridialytic SBP was shown to be increased ≥ 10 mm Hg in 63.3% and 18.8% of treatments in the piHTN and non-piHTN groups, respectively. Patients with piHTN were more likely to be of older age and have higher modified CCI or a higher rate of hypozincemia, whereas lower predialytic Hb, RBC, Hct, and level of blood zinc (all P < 0.05). There was no significant difference observed in the levels of other blood trace elements (copper, lead, manganese, and selenium), or other clinical or laboratory markers between the 2 groups at the time of enrollment in the study, or throughout the use of antihypertension drugs, or low calcium concentration dialysate, or dosage of rHuEPO, in the next 3 months.
Associations between Level of Blood Zinc and piHTN
Table 4 displays the results obtained from GLM models. Unadjusted analysis showed that with every mg/L point lower mean blood zinc, the peridialytic SBP change was increased by 4.524 mm Hg. After adjustment for age, preHD SBP, rHuEPO dosage, and modified CCI in model 2 or an adjustment of model 2 for gender and vintage (model 3), or after adjustment for age, BMI, smoking, gender, and vintage in model 4 or an adjustment of age, preHD SBP, rHuEPO dosage, CCI, BMI, and smoking (model 5), these findings were also corroborated. Furthermore, after adjusting for the abovementioned confounding factors, binary logistic regression in the GLM module of the obtained SPSS results showed that the lower levels of blood zinc at baseline or hypozincemia (defined by a level of blood zinc < 4.220 mg/L) were independently associated with piHTN, respectively (Table 5). No association was observed between neither of the other trace elements and piHTN, nor between other trace elements and peridialytic SBP change in univariate or multivariate analysis.
Discussion
After adjusting for confounding factors, our research showed for the first time an inverse association between the level of blood zinc and peridialytic SBP change, with lower blood zinc or hypozincemia being independently associated with piHTN in MHD patients.
As an important trace element and micronutrient, zinc has important structural, enzymatic, and regulatory functions, and is essential for the maintenance of a healthy body. Meta-analysis revealed that compared with healthy controls, lower levels of blood zinc or serum zinc were observed in MHD patients [17]; however, in the present study, the mean concentration of blood zinc was 5.46 ± 1.38 mg/L, similar with that reported in the general population with normal renal function [21]. Researchers have also found that 5.1–78% of MHD patients were hypozincemic [22,23,24], and this was also shown in our study where by using the cutoff by Cesbron et al. [21], we identified 29 patients (20.1%) with hypozincemia (blood zinc < 4.220 mg/L). Perhaps because of not only the use of ACE inhibitors or angiotensin 2 receptor antagonists or thiazide diuretics promoting zinc excretion with urine [25] but also because of decreased intake of zinc in diet, defective absorption of zinc in the intestine [26], and zinc removal in dialysate during HD, MHD patients with lower levels of blood zinc were more likely to be zinc deficient [27]. The rate of hypozincemia was much higher in the piHTN than in other groups (44.1 %), with this group also exhibiting the lower level of blood zinc (Table 3).
Hypozincemia might indicate zinc deficiency in MHD patients, but as the possible mechanisms of the lower levels of blood zinc or hypozincemia were independently associated with piHTN, this complicated the result. First, zinc deficiency has been reported to promote the activation of the hypoxia-inducible factor-1 (HIF-1)/ET-1 pathway, resulting in increased secretion of ET-1 and migration of endothelial cells [28]. Researchers noticed that the availability and function of endothelial NO were likely to be compromised if endothelial zinc stores became depleted in conditions of zinc deficiency [12], while interestingly, clinical studies showed that patients with iHTN had a significant decrease of the NO/ET-1 balance at the end of HD compared with control patients [9], potentially suggesting that zinc deficiency might play an important role in iHTN via the decreased NO/ET-1 balance. Second, through directly protecting easily oxidized groups, such as sulfhydryl and producing some other ultimate long-term antioxidants, such as metallothionein, zinc has been demonstrated to be involved in antioxidative actions. Furthermore, a meta-analysis showed that zinc supplementation could increase the levels of blood or serum zinc and lead to an anti-inflammatory and antioxidative effect in MHD patients [29]. As inflammation and oxidative stress have emerged as novel and major contributors to accelerated atherosclerosis [30], and atherosclerosis was considered to be one of the factors for iHTN [2], we therefore speculated that there might be some indirect connection between the lower levels of blood zinc and iHTN. Lastly, lower levels of blood zinc have been related to lower rHuEPO response and more dosage of rHuEPO for the treatment of anemia in MHD patients. Accordingly, oral zinc supplementation was shown to result in reduced erythropoietin responsiveness index [31], whereas intravenous rHuEPO caused an increase in the mean arterial pressure with an increase in ET-1 [32], which was related to iHTN. We also noticed that there were many studies focusing on the effects of zinc supplementation on MHD patients, but there was no study observing the relationship between zinc supplementation and iHTN.
Our study also reported that the piHTN group was characterized by older age, and higher CCI, whereas lower predialytic Hb, which were also corroborated in other studies with large and diverse populations [3, 5, 10, 33]. We observed that iHTN occurred in 90% of patients at least once in the 3 months of our follow-up, in accordance with other similar studies [10, 34]. However, our patients showed a higher prevalence of piHTN than that reported in previous studies [2, 5]. This could be explained on the basis that our patients were older (mean age 64.33 ± 13.39 years) than patients recruited in those studies [2, 3, 5, 10, 33], and patients with older age might be more likely to have piHTN [5].
Excess of potentially toxic trace elements and deficiency of essential trace elements are known to be common conditions in HD patients, and available data have suggested that levels of copper were higher, whereas levels of selenium, zinc, and manganese were lower in hemodialysis patients compared with controls [17]. Researchers observed that in HD patients, the decreased levels of serum selenium were correlated with diminished coronary flow reserve [35], and a higher ratio of serum copper/serum zinc implied increased cardiovascular risk [36]. However, we did not observe any significant differences in the levels of other blood trace elements (copper, manganese, selenium) between groups, nor any associations between any blood trace elements and piHTN or peridialytic SBP change in our study.
To a certain extent, the results of our study are strengthened because of the 3-month follow-up in assessing and defining piHTN. But admittedly, there have been a few limitations in our study. First, as this was a cross-sectional and observational study, and although we could note a possible protective effect of sufficient zinc in iHTN, no conclusions towards causation could be drawn. Second, multivariable models were performed after adjusting for many factors, but this study included a relatively small sample size. Therefore, our results should be cautiously interpreted due to a certain degree of unknown bias. Third, we conducted repeated measurements on blood pressure, but we only once tested the levels of blood zinc at the time of the enrollment of patients in the study. Fourth, we did not collect information on the source water used for HD or on the urine content of trace elements, residual kidney function, dietary intake, or extent of albuminuria, all of which could be associated with the level of blood zinc. Lastly, data related to endothelial dysfunction, such as plasma ET-1 and NO, were not recorded, and we acknowledge that these data might have been helpful in analyzing the relationship between the level of blood zinc and endothelial function.
Conclusions
Not much attention has been paid on the relationship between trace elements and iHTN, and hence, this is the first study demonstrating an association between lower levels of blood zinc and iHTN, after adjustment for confounding factors. We speculated that the underlying cause might be the negative effects of zinc deficiency on the vascular and endothelial function of MHD patients. Therefore, more attention should be paid to the levels of blood zinc for the management of iHTN in MHD patients, as iHTN has been associated with increased risk for hospitalizations and mortality. Prospective studies with larger sample size and with zinc supplementation are needed in the future in order to clarify the exact role of zinc in iHTN.
References
Dinesh K, Kunaparaju S, Cape K, Flythe JE, Feldman HI, Brunelli SM (2011) A model of systolic blood pressure during the course of dialysis and clinical factors associated with various blood pressure behaviors. Am J Kidney Dis 58(5):794–803
Georgianos PI, Sarafidis PA, Zoccali C (2015) Intradialysis hypertension in end-stage renal disease patients: clinical epidemiology, pathogenesis, and treatment. Hypertension 66(3):456–463
Nongnuch A, Campbell N, Stern E, El-Kateb S, Fuentes L, Davenport A (2015) Increased postdialysis systolic blood pressure is associated with extracellular overhydration in hemodialysis outpatients. Kidney Int 87(2):452–457
Inrig JK, Van Buren P, Kim C, Vongpatanasin W, Povsic TJ, Toto RD (2011) Intradialytic hypertension and its association with endothelial cell dysfunction. Clin J Am Soc Nephrol 6(8):2016–2024
Inrig JK, Oddone EZ, Hasselblad V, Gillespie B, Patel UD, Reddan D, Toto R, Himmelfarb J, Winchester JF, Stivelman J, Lindsay RM, Szczech LA (2007) Association of intradialytic blood pressure changes with hospitalization and mortality rates in prevalent ESRD patients. Kidney Int 71(5):454–461
Park J, Rhee CM, Sim JJ, Kim YL, Ricks J, Streja E, Vashistha T, Tolouian R, Kovesdy CP, Kalantar-Zadeh K (2013) A comparative effectiveness research study of the change in blood pressure during hemodialysis treatment and survival. Kidney Int 84(4):795–802
Agarwal R, Sinha AD, Pappas MK, Abraham TN, Tegegne GG (2014) Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrol Dial Transplant 29(3):672–681
Munoz MJ, Bayes LY, Sun S, Doss S, Schiller B (2011) Effect of lowering dialysate sodium concentration on interdialytic weight gain and blood pressure in patients undergoing thrice-weekly in-center nocturnal hemodialysis: a quality improvement study. Am J Kidney Dis 58(6):956–963
Chou KJ, Lee PT, Chen CL, Chiou CW, Hsu CY, Chung HM, Liu CP, Fang HC (2006) Physiological changes during hemodialysis in patients with intradialysis hypertension. Kidney Int 69(10):1833–1838
Meyring-Wosten A, Luo Y, Zhang H, Preciado P, Thijssen S, Wang Y, Kotanko P (2018) Intradialytic hypertension is associated with low intradialytic arterial oxygen saturation. Nephrol Dial Transplant 33(6):1040–1045
Mohammadifard N, Humphries KH, Gotay C, Mena-Sanchez G, Salas-Salvado J, Esmaillzadeh A, Ignaszewski A, Sarrafzadegan N (2019) Trace minerals intake: risks and benefits for cardiovascular health. Crit Rev Food Sci Nutr 59(8):1334–1346
Zalewski PD, Beltrame JF, Wawer AA, Abdo AI, Murgia C (2019) Roles for endothelial zinc homeostasis in vascular physiology and coronary artery disease. Crit Rev Food Sci Nutr 59(21):3511–3525
Alexanian I, Parissis J, Farmakis D, Athanaselis S, Pappas L, Gavrielatos G, Mihas C, Paraskevaidis I, Sideris A, Kremastinos D, Spiliopoulou C, Anastasiou-Nana M, Lekakis J, Filippatos G (2014) Clinical and echocardiographic correlates of serum copper and zinc in acute and chronic heart failure. Clin Res Cardiol 103(11):938–949
Benstoem C, Goetzenich A, Kraemer S, Borosch S, Manzanares W, Hardy G, Stoppe C (2015) Selenium and its supplementation in cardiovascular disease--what do we know? Nutrients 7(5):3094–3118
Lee YK, Lyu ES, Oh SY, Park HR, Ro HK, Heo YR, Hyun T, Choi MK (2015) Daily copper and manganese intakes and their relation to blood pressure in normotensive adults. Clin Nutr Res 4(4):259–266
Wu C, Woo JG, Zhang N (2017) Association between urinary manganese and blood pressure: results from National Health and Nutrition Examination Survey (NHANES), 2011-2014. Plos One 12(11):e188145
Tonelli M, Wiebe N, Hemmelgarn B, Klarenbach S, Field C, Manns B, Thadhani R, Gill J (2009) Trace elements in hemodialysis patients: a systematic review and meta-analysis. BMC Med 7:25
Gotch FA, Sargent JA (1985) A mechanistic analysis of the National Cooperative Dialysis Study (NCDS). Kidney Int 28(3):526–534
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Tonelli M, Wiebe N, Bello A, Field CJ, Gill JS, Hemmelgarn BR, Holmes DT, Jindal K, Klarenbach SW, Manns BJ, Thadhani R, Kinniburgh D (2018) Concentrations of trace elements and clinical outcomes in hemodialysis patients: a prospective cohort study. Clin J Am Soc Nephrol 13(6):907–915
Cesbron A, Saussereau E, Mahieu L, Couland I, Guerbet M, Goulle JP (2013) Metallic profile of whole blood and plasma in a series of 106 healthy volunteers. J Anal Toxiacol 37(7):401–405
Lee SH, Huang JW, Hung KY, Leu LJ, Kan YT, Yang CS, Chung WD, Huang CL, Chen PY, Chen JS, Chen WY (2000) Trace metals’ abnormalities in hemodialysis patients: relationship with medications. Artif Organs 24(11):841–844
Tonelli M, Wiebe N, Bello A, Field CJ, Gill JS, Hemmelgarn BR, Holmes DT, Jindal K, Klarenbach SW, Manns BJ, Thadhani R, Kinniburgh D (2017) Concentrations of trace elements in hemodialysis patients: a prospective cohort study. Am J Kideny Dis 70(5):696–704
Bogden JD, Zadzielski E, Weiner B, Oleske JM, Aviv A (1982) Release of some trace metals from disposable coils during hemodialysis. Am J Clin Nutr 36(3):403–409
Braun LA, Rosenfeldt F (2013) Pharmaco-nutrient interactions - a systematic review of zinc and antihypertensive therapy. Int J Clin Pract 67(8):717–725
Foote JW, Hinks LJ (1988) Zinc absorption in haemodialysis patients. Ann Clin Biochem 25(Pt 4):398–402
Rucker D, Thadhani R, Tonelli M (2010) Trace element status in hemodialysis patients. Semin Dial 23(4):389–395
Morand J, Briancon-Marjollet A, Lemarie E, Gonthier B, Arnaud J, Korichneva I, Godin-Ribuot D (2019) Zinc deficiency promotes endothelin secretion and endothelial cell migration through nuclear hypoxia-inducible factor-1 translocation. Am J Physiol Cell Physiol 317(2):C270–C276
Wang LJ, Wang MQ, Hu R, Yang Y, Huang YS, Xian SX, Lu L (2017) Effect of zinc supplementation on maintenance hemodialysis patients: a systematic review and meta-analysis of 15 randomized controlled trials. Biomed Res Int 2017:1024769
Liakopoulos V, Roumeliotis S, Gorny X, Dounousi E, Mertens PR (2017) Oxidative stress in hemodialysis patients: a review of the literature. Oxid Med Cell Longev 2017:3081856
Kobayashi H, Abe M, Okada K, Tei R, Maruyama N, Kikuchi F, Higuchi T, Soma M (2015) Oral zinc supplementation reduces the erythropoietin responsiveness index in patients on hemodialysis. Nutrients 7(5):3783–3795
Kang DH, Yoon KI, Han DS (1998) Acute effects of recombinant human erythropoietin on plasma levels of proendothelin-1 and endothelin-1 in haemodialysis patients. Nephrol Dial Transplant 13(11):2877–2883
Inrig JK, Patel UD, Toto RD, Szczech LA (2009) Association of blood pressure increases during hemodialysis with 2-year mortality in incident hemodialysis patients: a secondary analysis of the Dialysis Morbidity and Mortality Wave 2 Study. Am J Kidney Dis 54(5):881–890
Van Buren PN, Kim C, Toto RD, Inrig JK (2012) The prevalence of persistent intradialytic hypertension in a hemodialysis population with extended follow-up. Int J Artif Organs 35(12):1031–1038
Atakan A, Macunluoglu B, Kaya Y, Ari E, Demir H, Asicioglu E, Kaspar C (2013) Decreased serum selenium levels are correlated with diminished coronary flow reserve among hemodialysis patients. Biol Trace Elem Res 155(3):333–338
Reina DLTM, Navarro-Alarcon M, Del ML, Lopez-G DLSH, Palomares-Bayo M, Oliveras LM, Blanca HR, Agil A (2014) Serum Zn levels and Cu/Zn ratios worsen in hemodialysis patients, implying increased cardiovascular risk: a 2-year longitudinal study. Biol Trace Elem Res 158(2):129–135
Acknowledgments
The authors are thankful to all nurses in the hemodialysis center of Guangzhou Red Cross Hospital, Jinan University.
Funding
This research was supported by 2 grants from the Science and Technology Planning Project of Guangdong Province, 2014B030303002 and 2017B090904027, and 1 grant from the Guangzhou Municipal Health and Family Planning Commission: 20181A011020.
Author information
Authors and Affiliations
Contributions
Y. L. contributed to the study design, partial data collection, and drafting of the manuscript; Y. Z was involved in the analysis and interpretation of the data; L.W, X.Z., and D.Q collected BP data in every HD treatment; W.C followed up the patients; R.T and Y.L were involved in the study design, critical revision of the manuscript for important intellectual content, and final approval of the submitted manuscript.
Corresponding author
Ethics declarations
This study (including all protocols) was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Guangzhou Red Cross Hospital, Jinan University Hospital (license code: 2019-235-01).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Y., Zheng, Y., Wang, L. et al. Lower Levels of Blood Zinc Associated with Intradialytic Hypertension in Maintenance Hemodialysis Patients. Biol Trace Elem Res 199, 2514–2522 (2021). https://doi.org/10.1007/s12011-020-02385-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02385-4