Abstract
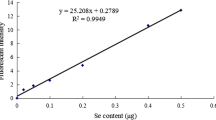
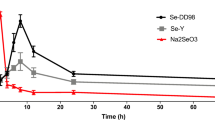
Selenium (Se) is an essential trace element for humans and animals. Appropriate amount of Se in the body can prevent a variety of diseases. However, Se deficiency leads to pathological changes such as skeletal muscle necrosis and pancreatic atrophy in livestock and poultry. Se preparations are widely used in the prevention and treatment of Se-deficient disease, but there is no unified standard of medication, and the safe dose range of Se is narrow. Therefore, it is of great significance to study the pharmacokinetics of low-Se ducklings and to formulate drug administration schemes. In the present study, eighty 1-day-old healthy ducklings were randomly selected, and fed with low-Se diet to 30 days of age (blood Se content ≦ 0.03 μg/mL). After the low Se duckling models were duplicated, blood samples and tissues of livers, pancreases, and thigh muscles were collected at different time points to detect Se content following oral administration of 0.1% sodium selenite (Na2SeO3) at 0.8 mg/kg BW, and the pharmacokinetics parameters were automatically calculated by MCPKP program. The results showed that pharmacokinetics characteristics of Na2SeO3 in blood, livers, and pancreases of ducklings were consistent with the first-order absorption and two-compartment open models; in thigh muscles was consistent with the first-order absorption and one compartment with a lag time open model. The primary kinetic parameters of Na2SeO3 in blood: the half-life of absorption was 5.9026 h, the time of reaching maximum concentration was 23.03 h, and the half-life of elimination was 131.13 h. The absorption of Na2SeO3 in livers was the quickest, pancreases and thigh muscles were in order of becoming slower, and the elimination of Na2SeO3 in thigh muscles was the quickest, livers and pancreases were in order of becoming slower. The administration parameters of multi-dose were calculated according to the kinetic of single-dose: loading dose (D*) was 1.7046 mg/kg BW, maintenance dose (D0) was 0.8 mg/kg BW, and dosing interval (τ) was 120 h. The results of this study can supplement and improve the theoretical system of Se metabolic kinetics, and provide experimental basis for the prevention and treatment of Se deficiency disease by rational drug use.


Similar content being viewed by others
References
Khoso PA, Zhang Y, Yin H, Teng X, Li S (2018) Selenium deficiency affects immune function by influencing selenoprotein and cytokine expression in chicken spleen. Biol Trace Elem Res. https://doi.org/10.1007/s12011-018-1396-9
Wang Y, Chen P, Zhao G, Sun K, Li D, Wan X, Zhang J (2015) Inverse relationship between elemental selenium nanoparticle size and inhibition of cancer cell growth in vitro and in vivo. Food Chem Toxicol 85:71–77. https://doi.org/10.1016/j.fct.2015.08.006
Gong T, Torres DJ, Berry MJ, Pitts MW (2018) Hypothalamic redox balance and leptin signaling - emerging role of selenoproteins. Free Radic Biol Med 127:172–181. https://doi.org/10.1016/j.freeradbiomed.2018.02.038
Sun X, Cui Y, Wang Q, Tang S, Cao X, Luo H, He Z, Hu X, Nie X, Yang Y, Wang T (2018) Proteogenomic analyses revealed favorable metabolism pattern alterations in rotifer Brachionus plicatilis fed with selenium-rich chlorella. J Agric Food Chem 66(26):6699–6707. https://doi.org/10.1021/acs.jafc.8b00139
Zheng S, Song H, Gao H, Liu C, Zhang Z, Fu J (2016) The antagonistic effect of selenium on lead-induced inflammatory factors and heat shock protein mRNA level in chicken cartilage tissue. Biol Trace Elem Res 173(1):177–184. https://doi.org/10.1007/s12011-016-0630-6
National Research Council (1998) Nutrient requirements of poultry, 10th edn. National Academy Press, Washington, DC
National Research Council (1998) Nutrient requirements of swine, 10th edn. National Academy Press, Washington, DC
Zheng SF, Bao RK, Zhang QJ, Wang SC, Lin HJ (2018) Endogenous hydrogen sulfide promotes apoptosis via mitochondrial pathways in the livers of broilers with selenium deficiency exudative diathesis disease. Biol Trace Elem Res 186:249–257. https://doi.org/10.1007/s12011-018-1292-3
Xu J, Wang L, Tang J, Jia G, Liu G, Chen X, Cai J, Shang H, Zhao H (2017) Pancreatic atrophy caused by dietary selenium deficiency induces hypoinsulinemic hyperglycemia via global down-regulation of selenoprotein encoding genes in broilers. PLoS One 12(8):e0182079. https://doi.org/10.1371/journal.pone.0182079
Del Bas JM, Rodriguez B, Puiggros F, Marine S, Rodriguez MA, Morina D, Armengol L, Caimari A, Arola L (2017) Hepatic accumulation of S-adenosylmethionine in hamsters with non-alcoholic-fatty liver disease associated to metabolic syndrome under selenium and vitamin E deficiency. Clin Sci. https://doi.org/10.1042/CS20171039
Oropeza-Moe M, Wisloff H, Bernhoft A (2015) Selenium deficiency associated porcine and human cardiomyopathies. J Trace Elem Med Biol 31:148–156. https://doi.org/10.1016/j.jtemb.2014.09.011
Sheppard AD, Blom L, Grant AB (1984) Levels of selenium in blood and tissues associated with some selenium deficiency diseases in New Zealand sheep. N Z Vet J 32(6):91–95. https://doi.org/10.1080/00480169.1984.35076
Surai PF, Fisinin VI (2015) Selenium in pig nutrition and reproduction: boars and semen quality-a review. Asian Australas J Anim Sci 28(5):730–746. https://doi.org/10.5713/ajas.14.0593
Zhan X, Qie Y, Wang M, Li X, Zhao R (2011) Selenomethionine: an effective selenium source for sow to improve se distribution, antioxidant status, and growth performance of pig offspring. Biol Trace Elem Res 142(3):481–491. https://doi.org/10.1007/s12011-010-8817-8
Dubreil C, Sainte Catherine O, Lalatonne Y, Journe C, Ou P, van Endert P, Motte L (2018) Tolerogenic iron oxide nanoparticles in type 1 diabetes: biodistribution and pharmacokinetics studies in nonobese diabetic mice. Small 14:e1802053. https://doi.org/10.1002/smll.201802053
Su J, Sripanidkulchai K, Suksamrarn A, Hu Y, Piyachuturawat P, Sripanidkulchai B (2012) Pharmacokinetics and organ distribution of diarylheptanoid phytoestrogens from Curcuma comosa in rats. J Nat Med 66(3):468–475. https://doi.org/10.1007/s11418-011-0607-x
Wang X, Wang WX (2017) Selenium induces the demethylation of mercury in marine fish. Environ Pollut 231(Pt 2):1543–1551. https://doi.org/10.1016/j.envpol.2017.09.014
Szulc-Musiol B, Gadomska-Nowak M, Danch A, Ryszka F (2004) Pharmacokinetics of selenium following oral administration selenium preparation in rabbits. Boll Chim Farm 143(2):62–64
Waldridge BM, Duran SH, Ravis WR, Paxton R, Herdt TH, Pugh DG (2004) Pharmacokinetics of subcutaneous selenium in adult llamas. Vet Ther 5(4):272–278
Blodgett DJ, Bevill RF (1987) Pharmacokinetics of selenium administered parenterally at toxic doses in sheep. Am J Vet Res 48(3):530–534
Ecimovic S, Velki M, Vukovic R, Stolfa Camagajevac I, Petek A, Bosnjakovic R, Grgic M, Engelmann P, Bodo K, Filipovic-Marijic V, Ivankovic D, Erk M, Mijosek T, Loncaric Z (2018) Acute toxicity of selenate and selenite and their impacts on oxidative status, efflux pump activity, cellular and genetic parameters in earthworm Eisenia andrei. Chemosphere 212:307–318. https://doi.org/10.1016/j.chemosphere.2018.08.095
Gobi N, Vaseeharan B, Rekha R, Vijayakumar S, Faggio C (2018) Bioaccumulation, cytotoxicity and oxidative stress of the acute exposure selenium in Oreochromis mossambicus. Ecotoxicol Environ Saf 162:147–159. https://doi.org/10.1016/j.ecoenv.2018.06.070
Zheng S, Jin X, Chen M, Shi Q, Zhang H, Xu S (2018) Hydrogen sulfide exposure induces jejunum injury via CYP450s/ROS pathway in broilers. Chemosphere 214:25–34. https://doi.org/10.1016/j.chemosphere.2018.09.002
Yang J, Zhang Y, Hamid S, Cai J, Liu Q, Li H, Zhao R, Wang H, Xu S, Zhang Z (2017) Interplay between autophagy and apoptosis in selenium deficient cardiomyocytes in chicken. J Inorg Biochem 170:17–25. https://doi.org/10.1016/j.jinorgbio.2017.02.006
Yao HD, Wu Q, Zhang ZW, Zhang JL, Li S, Huang JQ, Ren FZ, Xu SW, Wang XL, Lei XG (2013) Gene expression of endoplasmic reticulum resident selenoproteins correlates with apoptosis in various muscles of se-deficient chicks. J Nutr 143(5):613–619. https://doi.org/10.3945/jn.112.172395
Niyo Y, Glock RD, Ramsey FK, Ewan RC (1977) Effects of intramuscular injections of selenium and vitamin E on selenium-vitamin E deficiency in young pigs. Am J Vet Res 38(10):1479–1484
Fraga CG, Arias RF, Llesuy SF, Koch OR, Boveris A (1987) AEffect of vitamin E- and selenium-deficiency on rat liver chemiluminescence. Biochem J 242(2):383–386
Zhao X, Yao H, Fan R, Zhang Z, Xu S (2014) Selenium deficiency influences nitric oxide and selenoproteins in pancreas of chickens. Biol Trace Elem Res 161(3):341–349. https://doi.org/10.1007/s12011-014-0139-9
Huang JQ, Li DL, Zhao H, Sun LH, Xia XJ, Wang KN, Luo X, Lei XG (2011) The selenium deficiency disease exudative diathesis in chicks is associated with downregulation of seven common selenoprotein genes in liver and muscle. J Nutr 141(9):1605–1610. https://doi.org/10.3945/jn.111.145722
Haraya K, Tachibana T, Nezu J (2017) Quantitative prediction of therapeutic antibody pharmacokinetics after intravenous and subcutaneous injection in human. Drug Metab Pharmacokinet 32(4):208–217. https://doi.org/10.1016/j.dmpk.2017.05.002
Jastrzebski Z, Czyzewska-Szafran H, Remiszewska M, Fijalek Z, Fitak BA, Suchocki P (1997) Pharmacokinetics of selol, a new agent containing selenium, in rats. Drugs Exp Clin Res 23(1):7–11
Guenter W, Bragg DB (1977) Response of broiler chick to dietary selenium. Poult Sci 56(6):2031–2038
Saito Y, Takahashi K (2002) Characterization of selenoprotein P as a selenium supply protein. Eur J Biochem 269(22):5746–5751. https://doi.org/10.1046/j.1432-1033.2002.03298.x
Zhao Z, Barcus M, Kim J, Lum KL, Mills C, Lei XG (2016) High dietary selenium intake alters lipid metabolism and protein synthesis in liver and muscle of pigs. J Nutr 146(9):1625–1633. https://doi.org/10.3945/jn.116.229955
Lehr T, Narbe R, Jons O, Kloft C, Staab A (2010) Population pharmacokinetic modelling and simulation of single and multiple dose administration of meloxicam in cats. J Vet Pharmacol Ther 33(3):277–286. https://doi.org/10.1111/j.1365-2885.2009.01134.x
van Iersel M, Rossenu S, de Greef R, Waskin H (2018) A population pharmacokinetic model for a solid Oral tablet formulation of posaconazole. Antimicrob Agents Chemother 62(7). https://doi.org/10.1128/AAC.02465-17
Funding
This study was supported by the International (Regional) Cooperation and Exchange Projects of the National Natural Science Foundation of China (31320103920).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
All other Authors have read the manuscript and have agreed to submit it in its current form for consideration for publication in the Journal.
Rights and permissions
About this article
Cite this article
Zheng, S., Xing, H., Zhang, Q. et al. Pharmacokinetics of Sodium Selenite Administered Orally in Blood and Tissues of Selenium-Deficient Ducklings. Biol Trace Elem Res 190, 509–516 (2019). https://doi.org/10.1007/s12011-018-1567-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1567-8