Abstract
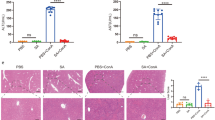
Hydrogen sulfide (H2S), an endogenous gasotransmitter, plays an important role in apoptosis. Exudative diathesis (ED) disease is associated with dietary selenium (Se) deficiency in broilers. The liver is one of the target organs of Se deficiency; however, little is known about the effect of H2S on apoptosis via mitochondrial pathways in the livers of broilers with ED disease. In the present study, we aimed to investigate the correlation between endogenous H2S and mitochondrial-mediated apoptosis in the livers of broilers with ED disease, as induced by Se deficiency. One hundred twenty healthy, 1-day-old broilers were randomly assigned to one of two groups (60 each) based on diet: Basal diet (control group, 0.2 mg/kg Se) or a low-Se diet (−Se group, 0.033 mg/kg Se). At day 20, 15 broilers of a similar weight were sacrificed from the control group, while the same number of broilers were euthanatized from the −Se group when displaying typical symptoms of ED between days 18 and 25. The livers were collected, and apoptosis was measured using a TUNEL assay. Additionally, H2S concentration, the expression of H2S synthases of cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS), and 3-mercaptopyruvate sulfurtransferase (3-MST), as well as mitochondrial apoptosis-related genes of Bcl-2, Bax, Bak, Cyt-C, Caspase-9, Caspase-3, and p53, were examined in livers. The results indicated that Se deficiency could induce apoptosis in the livers of broilers. Swelling, fractures, and vacuolization were visible in the mitochondrial cristae in the livers of the −Se group. The expression of H2S synthase-related genes and H2S concentration was significantly enhanced (P < 0.05) in the livers of the −Se group compared to controls. Moreover, a low-Se diet downregulated (P < 0.05) the level of Bcl-2 and upregulated (P < 0.05) the levels of Bax, Bak, Cyt-C, Caspase-9, Caspase-3, and p53. These results suggest that an H2S increase in the livers of ED broilers, which was induced by Se deficiency, is related to apoptosis mediated by mitochondrial pathways.






Similar content being viewed by others
References
Kohrle J, Gartner R (2009) Selenium and thyroid. Best Pract Res Clin Endocrinol Metab 23(6):815–827
Brown KM, Arthur JR (2001) Selenium, selenoproteins and human health: a review. Public Health Nutr 4(2b):593–599
Tabassum A, Bristow RG, Venkateswaran V (2010) Ingestion of selenium and other antioxidants during prostate cancer radiotherapy: a good thing? Cancer Treat Rev 36(3):230–234
Jiao X, Yang K, An Y, Teng X, Teng X (2017) Alleviation of lead-induced oxidative stress and immune damage by selenium in chicken bursa of Fabricius. Environ Sci Pollut Res Int 24(8):7555–7564
Hall JA, Vorachek WR, Stewart WC, Gorman ME, Mosher WD, Pirelli GJ, Bobe G (2013) Selenium supplementation restores innate and humoral immune responses in footrot-affected sheep. PLoS One 8(12):e82572
Huang JQ, Li DL, Zhao H, Sun LH, Xia XJ, Wang KN, Luo X, Lei XG (2011) The selenium deficiency disease exudative diathesis in chicks is associated with downregulation of seven common selenoprotein genes in liver and muscle. J Nutr 141(9):1605–1610
Mistry HD, Broughton Pipkin F, Redman CW, Poston L (2012) Selenium in reproductive health. Am J Obstet Gynecol 206(1):21–30
Yao L, Du Q, Yao H, Chen X, Zhang Z, Xu S (2015) Roles of oxidative stress and endoplasmic reticulum stress in selenium deficiency-induced apoptosis in chicken liver. Biometals 28(2):255–265
Turan B, Zaloglu N, Koc E, Saran Y, Akkas N (1997) Dietary selenium- and vitamin E-induced alterations in some rabbit tissues. Biol Trace Elem Res 58(3):237–253
Erkekoglu P, Zeybek ND, Giray BK, Rachidi W, Kizilgun M, Hininger-Favier I, Favier A, Asan E, Hincal F (2014) The effects of di(2-ethylhexyl)phthalate on rat liver in relation to selenium status. Int J Exp Pathol 95(1):64–77
Predmore BL, Lefer DJ, Gojon G (2012) Hydrogen sulfide in biochemistry and medicine. Antioxid Redox Signal 17(1):119–140
Guan Q, Zhang Y, Yu C, Liu Y, Gao L, Zhao J (2012) Hydrogen sulfide protects against high-glucose-induced apoptosis in endothelial cells. J Cardiovasc Pharmacol 59(2):188–193
Cuevasanta E, Moller MN, Alvarez B (2016) Biological chemistry of hydrogen sulfide and persulfides. Arch Biochem Biophys 617:9–25. https://doi.org/10.1016/j.abb.2016.09.018
Giles S, Boehm P, Brogan C, Bannigan J (2008) The effects of ethanol on CNS development in the chick embryo. Reprod Toxicol 25:224–230
Kobayashi C, Yaegaki K, Calenic B, Ishkitiev N, Imai T, Ii H, Aoyama I, Kobayashi H, Izumi Y, Haapasalo M (2011) Hydrogen sulfide causes apoptosis in human pulp stem cells. J Endod 37(4):479–484
Bekpinar S, Develi-Is S, Unlucerci Y, Kusku-Kiraz Z, Uysal M, Gurdol F (2013) Modulation of arginine and asymmetric dimethylarginine concentrations in liver and plasma by exogenous hydrogen sulfide in LPS-induced endotoxemia. Can J Physiol Pharmacol 91(12):1071–1075
Zong Y, Huang Y, Chen S, Zhu M, Chen Q, Feng S, Sun Y, Zhang Q, Tang C, Du J, Jin H (2015) Downregulation of endogenous hydrogen sulfide pathway is involved in mitochondrion-related endothelial cell apoptosis induced by high salt. Oxidative Med Cell Longev 2015:754670
Liu C, Fu J, Liu C, Li S (2015) The role of nitric oxide and autophagy in liver injuries induced by selenium deficiency in chickens. RSC Adv 5(62):50549–50556
Du JT, Li W, Yang JY, Tang CS, Li Q, Jin HF (2013) Hydrogen sulfide is endogenously generated in rat skeletal muscle and exerts a protective effect against oxidative stress. Chin Med J 126(5):930–936
Xu JX, Cao CY, Sun YC, Wang LL, Li N, Xu SW, Li JL (2014) Effects on liver hydrogen peroxide metabolism induced by dietary selenium deficiency or excess in chickens. Biol Trace Elem Res 159(1–3):174–182
Hostetler CE, Kincaid RL (2004) Maternal selenium deficiency increases hydrogen peroxide and total lipidperoxides in porcine fetal liver. Biol Trace Elem Res 97(1):43–56
Wang X, An Y, Jiao W, Zhang Z, Han H, Gu X, Teng X (2017) Selenium protects against lead-induced apoptosis via endoplasmic reticulum stress in chicken kidneys. Biol Trace Elem Res https://doi.org/10.1007/s12011-017-1097-9
Yao HD, Wu Q, Zhang ZW, Zhang JL, Li S, Huang JQ, Ren FZ, Xu SW, Wang XL, Lei XG (2013) Gene expression of endoplasmic reticulum resident selenoproteins correlates with apoptosis in various muscles of se-deficient chicks. J Nutr 143(5):613–619
Zhang ZW, Zhang JL, Gao YH, Wang QH, Li S, Wang XL, Xu SW (2013) Effect of oxygen free radicals and nitric oxide on apoptosis of immune organ induced by selenium deficiency in chickens. Biometals 26(2):355–365
Moore PK, Bhatia M, Moochhala S (2003) Hydrogen sulfide: from the smell of the past to the mediator of the future? Trends Pharmacol Sci 24(12):609–611
Yan J, Teng F, Chen W, Ji Y, Gu Z (2012) Cystathionine beta-synthase-derived hydrogen sulfide regulates lipopolysaccharide-induced apoptosis of the BRL rat hepatic cell line in vitro. Exp Ther Med 4(5):832–838
Zhao W, Ndisang JF, Wang R (2003) Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol 81(9):848–853
Mani S, Cao W, Wu L, Wang R (2014) Hydrogen sulfide and the liver. Nitric Oxide 41:62–71
Hwang SY, Sarna LK, Siow YL, O K (2013) High-fat diet stimulates hepatic cystathionine beta-synthase and cystathionine gamma-lyase expression. Can J Pjysiol Phharmacol 91(11):913–919
Huang B, Chen CT, Chen CS, Wang YM, Hsieh HJ, Wang DL (2015) Laminar shear flow increases hydrogen sulfide and activates a nitric oxide producing signaling cascade in endothelial cells. Biochem Biophys Res Commun 464(4):1254–1259
Fiorucci S, Antonelli E, Mencarelli A, Orlandi S, Renga B, Rizzo G, Distrutti E, Shah V, Morelli A (2005) The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology 42(3):539–548
Yan Y, Chen C, Zhou H, Gao H, Chen L, Chen L, Gao L, Zhao R, Sun Y (2013) Endogenous hydrogen sulfide formation mediates the liver damage in endotoxemic rats. Res Vet Sci 94(3):590–595
Yang G, Sun X, Wang R (2004) Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J 18(14):1782–1784
Yao HD, Wu Q, Zhang ZW, Li S, Wang XL, Lei XG, Xu SW (2013) Selenoprotein W serves as an antioxidant in chicken myoblasts. Biochim Biophys Acta 1830(4):3112–3120
Kaushal N, Bansal MP (2007) Dietary selenium variation-induced oxidative stress modulates CDC2/cyclin B1 expression and apoptosis of germ cells in mice testis. J Nutr Biochem 18(8):553–564
Chen TF, Wong YS, Zheng WJ, Liu J (2009) Caspase- and p53-dependent apoptosis in breast carcinoma cells induced by a synthetic selenadiazole derivative. Chem Biol Interact 180(1):54–60
Green DR, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science 305(5684):626–629
Al-Fatlawi AA, Al-Fatlawi AA, Irshad M, Zafaryab M, Alam Rizvi MM, Ahmad A (2014) Rice bran phytic acid induced apoptosis through regulation of Bcl-2/Bax and p53 genes in HepG2 human hepatocellular carcinoma cells. Asian Pac J Cancer Prev 15(8):3731–3736
Yang G, Yang W, Wu L, Wang R (2007) H2S, endoplasmic reticulum stress, and apoptosis of insulin-secreting beta cells. J Biol Chem 282(22):16567–16576
Acknowledgments
This study was supported by the International (Regional) Cooperation and Exchange Projects of the National Natural Science Foundation of China (31320103920), the National Natural Science Foundation of China (31272626), the National Natural Science Foundation of China (31502134), and the University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (UNPYSCT-2016002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were performed in an animal facility that was accredited by the Institutional Animal Care and Use Committee of Northeast Agricultural University and approved under protocol number SRM-11
Rights and permissions
About this article
Cite this article
Zheng, Sf., Bao, Rk., Zhang, Qj. et al. Endogenous Hydrogen Sulfide Promotes Apoptosis via Mitochondrial Pathways in the Livers of Broilers with Selenium Deficiency Exudative Diathesis Disease. Biol Trace Elem Res 186, 249–257 (2018). https://doi.org/10.1007/s12011-018-1292-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1292-3