Abstract
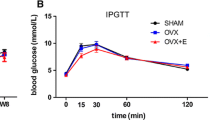
Investigations of bone mass and marrow adiposity are critical for defining the role of zinc (Zn) in bone metabolism. Rats used for study were grouped as follows: control (sham), ovariectomy (OVX), ovariectomy + estradiol (OVX-E), ovariectomy + Zn treatment (OVX-Zn). Bone mineral density (BMD) was quantified (microCT); serum osteocalcin, adiponectin, RANKL, and TRAP levels were assayed (ELISA); and biochemical determinations of serum alkaline phosphatase (ALP), calcium (Ca), and phosphorus (P) were done. Cells derived from bone mesenchymal stem cell (BMSC) isolates of respective test groups were compared, identifying primary osteoblasts by MTT assay and adipocytes by Oil Red O stain. Osteocalcin and adiponectin levels in culture supernatants were determined by ELISA. Zn supplementation resulted in a modest increase in BMD, but serum osteocalcin and ALP activity increased significantly (P < 0.01, both). Serum levels of RANKL and TRAP were lower in OVX-Zn (vs OVX) rats (P < 0.01), whereas serum concentrations of adiponectin, Ca, and P did not differ by group. Osteocalcin level was significantly upregulated ex vivo (P < 0.01) in the supernatant of cultured OVX-Zn (vs OVX) cells, accompanied by a slight upturn in osteoblastic differentiation. However, Oil Red O uptake and adiponectin level in supernatant were sharply diminished in cultured OVX-Zn (vs OVX) cells (P < 0.01). Overall, we concluded that Zn contributes to bone mass by marginally stimulating differentiation and proliferation of osteoblasts and by effectively inhibiting osteoclastic and adipocytic differentiation of BMSCs.







Similar content being viewed by others
References
Haumont S (1961) Distribution of zinc in bone tissues. J Histochem Cytochem 9(2):141–145
Aaseth J, Boivin G, Andersen O (2012) Osteoporosis and trace elements—an overview. J Trace Elem Med Biol 26(2–3):149–152
Yamaguchi M, Oishi H, Suketa Y (1987) Stimulatory effect of zinc on bone formation in tissue culture. Biochem Pharmacol 36(22):4007–4012
Seo HJ, Cho YE, Kim T, Shin HI, Kwun IS (2010) Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr Res Pract 4(5):356–361
Eberle J, Schmidmayer S, Erben RG, Stangassinger M, Roth HP (1999) Skeletal effects of zinc deficiency in growing rats. J Trace Elem Med Biol 13(1–2):21–26
Liang D, Yang M, Guo B, Cao J, Yang L, Guo X (2012) Zinc upregulates the expression of osteoprotegerin in mouse osteoblasts MC3T3-E1 through PKC/MAPKpathways. Biol Trace Elem Res 146(3):340–348
Li BB, Yu SF (2006) Effect of extracellular zinc on osteoclastic resorption in dental mineralized tissues. J Peking Univ Health Sci 38(6):644–647
Yamaguchi M, Uchiyama S (2004) Receptor activator of NF-kappa B ligand stimulated osteoclastogenesis in mouse marrow culture is suppressed by zinc in vitro. Int J Mol Med 14(1):81–85
Hie M, Tsukamoto I (2011) Administration of zinc inhibits osteoclastogenesis through the suppression of RANK expression in bone. Eur J Pharmacol 668(1–2):140–146
Park KH, Park B, Yoon DS et al (2013) Zinc inhibits osteoclast differentiation by suppression of Ca2 + −Calcineurin-NFATc1 signaling pathway. Cell Commun Signal. doi:10.1186/1478-811X-11-74
Razmandeh R, Nasli-Esfahani E, Heydarpour R et al (2014) Association of zinc, copper and magnesium with bone mineral density in Iranian postmenopausal women—a casecontrol study. J Diabetes Metab Disord 13(1):43. doi:10.1186/2251-6581-13-43
Yamaguchi M, Weitzmann MN (2011) Zinc stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-κB activation. Mol Cell Biochem 355(1–2):179–186
New SA (1997) Nutritional influences on bone mineral density: a cross-sectional study in premenopausal women. Am J Clin Nutr 65(6):1831–1839
Bhardwaj P, Rai DV, Garg ML (2013) Zinc as a nutritional approach to bone loss prevention in an ovariectomized rat model. Menopause 20(11):1184–1193
Atkinson RL, Dahms WT, Bray GA, Jacob R, Sandstead HH (1978) Plasma zinc and copper in obesity and after intestinal bypass. Ann Intern Med 89(4):491–493
Di Martino G, Matera MG, De Martino B, Vacca C, Di Martino S, Rossi F (1993) Relationship between zinc and obesity. J Med 24(2–3):177–183
Marreiro DN, Fisberg M, Cozzolino SM (2002) Zinc nutritional status in obese children and adolescents. Biol Trace Elem Res 86(2):107–122
Liu MJ, Bao S, Bolin ER et al (2013) Zinc deficiency augments leptin production and exacerbates macrophage infiltration into adipose tissue in mice fed a high-fat diet. J Nutr 143(7):1036–1045
Patsch JM, Li X, Baum T et al (2013) Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res 28(8):1721–1728
Georgiou KR, Hui SK, Xian CJ (2012) Regulatory pathways associated with bone loss and bone marrow adiposity caused by aging, chemotherapy, glucocorticoid therapy and radiotherapy. Am J Stem Cells 1(3):205–224
Morita Y, Iwamoto I, Mizuma N et al (2006) Precedence of the shift of body-fat distribution over the change in body composition after menopause. J Obstet Gynaecol Res 32(5):513–516
Pittenger MF, Mackay AM, Beck SC et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147
Taisum HH, Elizabeth BC, David BM (2004) Zinc intakes and plasma concentrations in men with osteoporosis: the Rancho Bernardo Study. Am J Clin Nutr 80(3):715–772
Cao T, Shirota T, Yamazaki M, Ohno K, Michi KI (2001) Bone mineral density in mandibles of ovariectomized rabbits. Clin Oral Implants Res 12(6):604–608
Siris ES, Boonen S, Mitchell PJ, Bilezikian J, Silverman S (2012) What’s in a name? What constitutes the clinical diagnosis of osteoporosis? Osteoporos Int 23(8):2093–2097
Bancroft GN, Sikavitsas VI, van den Dolder J et al (2002) Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci U S A 99(20):12600–12605
DiGirolamo CM, Stokes D, Colter DG, Phinney DG, Class R, Prockop DJ (1999) Propagation and senescence of human marrow stromal cells in culture—a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol 107(2):275–281
Rozman C, Feliu E, Berga L, Reverter JC, Climent C, Ferran MJ (1989) Age-related variations of fat tissue fraction in normal human bone marrow depend both on size and number of adipocytes: a stereological study. Exp Hematol 17(1):34–37
Li GW, Xu Z, Chang SX, Nian H, Wang XY, Qin LD (2014) Icariin prevents ovariectomy-induced bone loss and lowers marrow adipogenesis. Menopause 21(9):1007–1016
Menagh PJ, Turner RT, Jump DB et al (2010) Growth hormone regulates the balance between bone formation and bone marrow adiposity. J Bone Miner Res 25(4):757–768
Hirota K, Morikawa K, Hanada H et al (2010) Effect of genistein and daidzein on the proliferation and differentiation of human preadipocyte cell line. J Agric Food Chem 58(9):5821–5827
Lee OH, Seo DH, Park CS, Kim YC (2010) Puerarin enhances adipocyte differentiation, adiponectin expression, and antioxidant response in 3T3-L1 cells. Biofactors 36(6):459–467
Acknowledgments
This research was funded by National Natural Science Foundations of China, Beijing, China (No. 30901671).
Conflict of interest
The authors certify that there is no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, B., Liu, H. & Jia, S. Zinc Enhances Bone Metabolism in Ovariectomized Rats and Exerts Anabolic Osteoblastic/Adipocytic Marrow Effects Ex Vivo. Biol Trace Elem Res 163, 202–207 (2015). https://doi.org/10.1007/s12011-014-0185-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0185-3