Abstract
Background
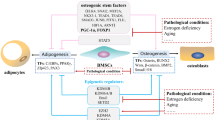
Accumulation of bone marrow adipose tissue (BMAT) is always seen in osteoporosis induced by estrogen deficiency. Herein, we aimed to investigate the mechanisms and consequences of this phenomenon by establishing a mouse model of osteoporosis caused by ovariectomy (OVX)-mimicked estrogen deficiency.
Methods
Micro-CT, osmium tetroxide staining, and histological analyses were performed to examine the changes in bone microstructure, BMAT and white adipose tissue (WAT) in OVX mice compared to sham mice. The osteogenesis and adipogenesis of primary bone marrow stromal cells (BMSCs) isolated from sham and OVX mice were compared in vitro. The molecular phenotypes of BMAT and WAT were determined and compared by quantitative PCR (qPCR). Bone marrow adipocyte-conditioned medium (BMA CM) was prepared from sham or OVX mice for coculture assays, and BMSCs or bone marrow monocytes/macrophages (BMMs) were isolated and subjected to osteoblast and osteoclast differentiation, respectively. Cell staining and qPCR were used to assess the effects of BMAT on bone metabolism.
Results
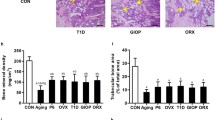
OVX-induced estrogen deficiency induced reductions in both cortical and trabecular bone mass along with an expansion of BMAT volume. At the cellular level, loss of estrogen inhibited BMSC osteogenesis and promoted BMSC adipogenesis, whereas addition of estradiol exerted the opposite effects. In response to estrogen deficiency, despite the common proinflammatory molecular phenotype observed in both fat depots, BMAT, unlike WAT, unexpectedly exhibited an increase in adipocyte differentiation and lipolytic activity as well as the maintenance of insulin sensitivity. Importantly, BMAT, but not WAT, presented increased mRNA levels of both BMP receptor inhibitors (Grem1, Chrdl1) and Rankl following OVX. In addition, treatment with BMA CM, especially from OVX mice, suppressed the osteoblast differentiation of BMSCs while favoring the osteoclast differentiation of BMMs.
Conclusion
Our study illustrates that OVX-induced estrogen deficiency results in bone loss and BMAT expansion by triggering imbalance between the osteogenesis and adipogenesis of BMSCs. Furthermore, expanded BMAT, unlike typical WAT, may negatively regulate bone homeostasis through paracrine inhibition of osteoblast-mediated bone formation and promotion of osteoclast-mediated bone resorption.






Similar content being viewed by others
References
B.J. Kim, J.M. Koh, Coupling factors involved in preserving bone balance. Cell Mol. Life Sci. 76(7), 1243–1253 (2019). https://doi.org/10.1007/s00018-018-2981-y
D.M. Black, C.J. Rosen, Clinical practice. postmenopausal osteoporosis. N. Engl. J. Med. 374(3), 254–262 (2016). https://doi.org/10.1056/NEJMcp1513724
B. Langdahl, Treatment of postmenopausal osteoporosis with bone-forming and antiresorptive treatments: combined and sequential approaches. Bone 139, 115516 (2020). https://doi.org/10.1016/j.bone.2020.115516
M. Lorentzon, Treating osteoporosis to prevent fractures: current concepts and future developments. J. Intern. Med. 285(4), 381–394 (2019). https://doi.org/10.1111/joim.12873
S.D. Mistry, G.N. Woods, S. Sigurdsson, S.K. Ewing, T.F. Hue, G. Eiriksdottir, K. Xu, J.F. Hilton, D.M. Kado, V. Gudnason, T.B. Harris, C.J. Rosen, T.F. Lang, X. Li, A.V. Schwartz, Sex hormones are negatively associated with vertebral bone marrow fat. Bone 108, 20–24 (2018). https://doi.org/10.1016/j.bone.2017.12.009
Milisic, L., Vegar-Zubovic, S., Valjevac, A.: Bone marrow adiposity is inversely associated with bone mineral density in postmenopausal females. Med. Glas (Zenica). 17(1), (2020). https://doi.org/10.17392/1053-20.
K.M. Beekman, M. Zwaagstra, A.G. Veldhuis-Vlug, H.W. van Essen, M. den Heijer, M. Maas, G. Kerckhofs, T.N. Parac-Vogt, P.H. Bisschop, N. Bravenboer, Ovariectomy increases RANKL protein expression in bone marrow adipocytes of C3H/HeJ mice. Am. J. Physiol. Endocrinol. Metab. 317(6), E1050–E1054 (2019). https://doi.org/10.1152/ajpendo.00142.2019
S. Li, H. Jiang, B. Wang, M. Gu, N. Zhang, W. Liang, Y. Wang, Effect of leptin on marrow adiposity in ovariectomized rabbits assessed by proton magnetic resonance spectroscopy. J. Comput. Assist. Tomogr. 42(4), 588–593 (2018). https://doi.org/10.1097/RCT.0000000000000725
E.J. Limonard, A.G. Veldhuis-Vlug, L. van Dussen, J.H. Runge, M.W. Tanck, E. Endert, A.C. Heijboer, E. Fliers, C.E. Hollak, E.M. Akkerman, P.H. Bisschop, Short-term effect of estrogen on human bone marrow fat. J. Bone Min. Res. 30(11), 2058–2066 (2015). https://doi.org/10.1002/jbmr.2557
A. Elbaz, D. Rivas, G. Duque, Effect of estrogens on bone marrow adipogenesis and Sirt1 in aging C57BL/6J mice. Biogerontology 10(6), 747–755 (2009). https://doi.org/10.1007/s10522-009-9221-7
K.M. Beekman, A.G. Veldhuis-Vlug, M. den Heijer, M. Maas, A.M. Oleksik, M.W. Tanck, S.M. Ott, R.J. van ‘t Hof, P. Lips, P.H. Bisschop, N. Bravenboer, The effect of raloxifene on bone marrow adipose tissue and bone turnover in postmenopausal women with osteoporosis. Bone 118, 62–68 (2019). https://doi.org/10.1016/j.bone.2017.10.011
Y. Yang, X. Luo, F. Yan, Z. Jiang, Y. Li, C. Fang, J. Shen, Effect of zoledronic acid on vertebral marrow adiposity in postmenopausal osteoporosis assessed by MR spectroscopy. Skelet. Radio. 44(10), 1499–1505 (2015). https://doi.org/10.1007/s00256-015-2200-y
Y. Yang, X. Luo, X. Xie, F. Yan, G. Chen, W. Zhao, Z. Jiang, C. Fang, J. Shen, Influences of teriparatide administration on marrow fat content in postmenopausal osteopenic women using MR spectroscopy. Climacteric 19(3), 285–291 (2016). https://doi.org/10.3109/13697137.2015.1126576
de Paula, F.J.A., Rosen, C.J.: Marrow adipocytes: origin, structure, and function. Annu. Rev. Physiol. (2019). https://doi.org/10.1146/annurev-physiol-021119-034513
W.P. Cawthorn, E.L. Scheller, B.S. Learman, S.D. Parlee, B.R. Simon, H. Mori, X. Ning, A.J. Bree, B. Schell, D.T. Broome, S.S. Soliman, J.L. DelProposto, C.N. Lumeng, A. Mitra, S.V. Pandit, K.A. Gallagher, J.D. Miller, V. Krishnan, S.K. Hui, M.A. Bredella, P.K. Fazeli, A. Klibanski, M.C. Horowitz, C.J. Rosen, O.A. MacDougald, Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 20(2), 368–375 (2014). https://doi.org/10.1016/j.cmet.2014.06.003
M. Tencerova, F. Figeac, N. Ditzel, H. Taipaleenmaki, T.K. Nielsen, M. Kassem, High-fat diet-induced obesity promotes expansion of bone marrow adipose tissue and impairs skeletal stem cell functions in mice. J. Bone Min. Res. 33(6), 1154–1165 (2018). https://doi.org/10.1002/jbmr.3408
E.L. Scheller, N. Troiano, J.N. Vanhoutan, M.A. Bouxsein, J.A. Fretz, Y. Xi, T. Nelson, G. Katz, R. Berry, C.D. Church, C.R. Doucette, M.S. Rodeheffer, O.A. Macdougald, C.J. Rosen, M.C. Horowitz, Use of osmium tetroxide staining with microcomputerized tomography to visualize and quantify bone marrow adipose tissue in vivo. Methods Enzymol. 537, 123–139 (2014). https://doi.org/10.1016/B978-0-12-411619-1.00007-0
Y. Fan, J.I. Hanai, P.T. Le, R. Bi, D. Maridas, V. DeMambro, C.A. Figueroa, S. Kir, X. Zhou, M. Mannstadt, R. Baron, R.T. Bronson, M.C. Horowitz, J.Y. Wu, J.P. Bilezikian, D.W. Dempster, C.J. Rosen, B. Lanske, Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab. 25(3), 661–672 (2017). https://doi.org/10.1016/j.cmet.2017.01.001
F. Liu, Y. Yuan, L. Bai, L. Yuan, L. Li, J. Liu, Y. Chen, Y. Lu, J. Cheng, J. Zhang, LRRc17 controls BMSC senescence via mitophagy and inhibits the therapeutic effect of BMSCs on ovariectomy-induced bone loss. Redox Biol. 43, 101963 (2021). https://doi.org/10.1016/j.redox.2021.101963
A. Rauch, A.K. Haakonsson, J.G.S. Madsen, M. Larsen, I. Forss, M.R. Madsen, E.L. Van Hauwaert, C. Wiwie, N.Z. Jespersen, M. Tencerova, R. Nielsen, B.D. Larsen, R. Rottger, J. Baumbach, C. Scheele, M. Kassem, S. Mandrup, Osteogenesis depends on commissioning of a network of stem cell transcription factors that act as repressors of adipogenesis. Nat. Genet. 51(4), 716–727 (2019). https://doi.org/10.1038/s41588-019-0359-1
N. Saleh, N.A. Nassef, M.K. Shawky, M.I. Elshishiny, H.A. Saleh, Novel approach for pathogenesis of osteoporosis in ovariectomized rats as a model of postmenopausal osteoporosis. Exp. Gerontol. 137, 110935 (2020). https://doi.org/10.1016/j.exger.2020.110935
Woods, G.N., Ewing, S.K., Sigurdsson, S., Kado, D.M., Eiriksdottir, G., Gudnason, V., Hue, T.F., Lang, T.F., Vittinghoff, E., Harris, T.B., Rosen, C., Xu, K., Li, X., Schwartz, A.V.: Greater bone marrow adiposity predicts bone loss in older women. J. Bone Miner. Res. (2019). https://doi.org/10.1002/jbmr.3895
D. Krishnamoorthy, D.M. Frechette, B.J. Adler, D.E. Green, M.E. Chan, C.T. Rubin, Marrow adipogenesis and bone loss that parallels estrogen deficiency is slowed by low-intensity mechanical signals. Osteoporos. Int. 27(2), 747–756 (2016). https://doi.org/10.1007/s00198-015-3289-5
Q. Liu, X. Zhang, Y. Jiao, X. Liu, Y. Wang, S.L. Li, W. Zhang, F.M. Chen, Y. Ding, C. Jiang, Z. Jin, In vitro cell behaviors of bone mesenchymal stem cells derived from normal and postmenopausal osteoporotic rats. Int. J. Mol. Med. 41(2), 669–678 (2018). https://doi.org/10.3892/ijmm.2017.3280
B. Gao, Q. Huang, Q. Jie, L. Wang, H.Y. Zhang, J. Liu, L. Yang, Z.J. Luo, Dose-response estrogen promotes osteogenic differentiation via GPR40 (FFAR1) in murine BMMSCs. Biochimie 110, 36–44 (2015). https://doi.org/10.1016/j.biochi.2015.01.001
Puolakkainen, T., Rummukainen, P., Pihala-Nieminen, V., Ritvos, O., Savontaus, E., Kiviranta, R.: Treatment with soluble activin Type IIB receptor ameliorates ovariectomy-induced bone loss and fat gain in mice. Calcif. Tissue Int. (2022). https://doi.org/10.1007/s00223-021-00934-0
J. Medina-Contreras, R. Villalobos-Molina, A. Zarain-Herzberg, J. Balderas-Villalobos, Ovariectomized rodents as a menopausal metabolic syndrome model. A minireview. Mol. Cell Biochem. 475(1-2), 261–276 (2020). https://doi.org/10.1007/s11010-020-03879-4
Sharma, D.K., Anderson, P.H., Morris, H.A., Clifton, P.M.: Visceral fat is a negative determinant of bone health in obese postmenopausal women. Int. J. Environ. Res. Public Health 17(11), (2020). https://doi.org/10.3390/ijerph17113996
C.T. Liu, K.E. Broe, Y. Zhou, S.K. Boyd, L.A. Cupples, M.T. Hannan, E. Lim, R.R. McLean, E.J. Samelson, M.L. Bouxsein, D.P. Kiel, Visceral adipose tissue is associated with bone microarchitecture in the framingham osteoporosis study. J. Bone Miner. Res. 32(1), 143–150 (2017). https://doi.org/10.1002/jbmr.2931
J. Pfeilschifter, R. Koditz, M. Pfohl, H. Schatz, Changes in proinflammatory cytokine activity after menopause. Endocr. Rev. 23(1), 90–119 (2002). https://doi.org/10.1210/edrv.23.1.0456
S. Almuraikhy, W. Kafienah, M. Bashah, I. Diboun, M. Jaganjac, F. Al-Khelaifi, H. Abdesselem, N.A. Mazloum, M. Alsayrafi, V. Mohamed-Ali, M.A. Elrayess, Interleukin-6 induces impairment in human subcutaneous adipogenesis in obesity-associated insulin resistance. Diabetologia 59(11), 2406–2416 (2016). https://doi.org/10.1007/s00125-016-4031-3
J.R. Acosta, I. Douagi, D.P. Andersson, J. Backdahl, M. Ryden, P. Arner, J. Laurencikiene, Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia 59(3), 560–570 (2016). https://doi.org/10.1007/s00125-015-3810-6
M. Tencerova, M. Frost, F. Figeac, T.K. Nielsen, D. Ali, J.L. Lauterlein, T.L. Andersen, A.K. Haakonsson, A. Rauch, J.S. Madsen, C. Ejersted, K. Hojlund, M. Kassem, Obesity-associated hypermetabolism and accelerated senescence of bone marrow stromal stem cells suggest a potential mechanism for bone fragility. Cell Rep. 27(7), 2050–2062.e6 (2019). https://doi.org/10.1016/j.celrep.2019.04.066
Zou, W., Rohatgi, N., Brestoff, J.R., Li, Y., Barve, R.A., Tycksen, E., Kim, Y., Silva, M.J., Teitelbaum, S.L.: Ablation of fat cells in adult mice induces massive bone gain. Cell Metab. (2020). https://doi.org/10.1016/j.cmet.2020.09.011
Yu, W., Zhong, L., Yao, L., Wei, Y., Gui, T., Li, Z., Kim, H., Holdreith, N., Jiang, X., Tong, W., Dyment, N., Liu, X.S., Yang, S., Choi, Y., Ahn, J., Qin, L.: Bone marrow adipogenic lineage precursors promote osteoclastogenesis in bone remodeling and pathologic bone loss. J. Clin. Invest. 131(2), (2021). https://doi.org/10.1172/JCI140214
S. Takeshita, T. Fumoto, Y. Naoe, K. Ikeda, Age-related marrow adipogenesis is linked to increased expression of RANKL. J. Biol. Chem. 289(24), 16699–16710 (2014). https://doi.org/10.1074/jbc.M114.547919
D. Ferland-McCollough, D. Maselli, G. Spinetti, M. Sambataro, N. Sullivan, A. Blom, P. Madeddu, MCP-1 feedback loop between adipocytes and mesenchymal stromal cells causes fat accumulation and contributes to hematopoietic stem cell rarefaction in the bone marrow of patients with diabetes. Diabetes 67(7), 1380–1394 (2018). https://doi.org/10.2337/db18-0044
S. Zhu, H. He, C. Gao, G. Luo, Y. Xie, H. Wang, L. Tian, X. Chen, X. Yu, C. He, Ovariectomy-induced bone loss in TNFalpha and IL6 gene knockout mice is regulated by different mechanisms. J. Mol. Endocrinol. 60(3), 185–198 (2018). https://doi.org/10.1530/JME-17-0218
L. Thommesen, A.K. Stunes, M. Monjo, K. Grosvik, M.V. Tamburstuen, E. Kjobli, S.P. Lyngstadaas, J.E. Reseland, U. Syversen, Expression and regulation of resistin in osteoblasts and osteoclasts indicate a role in bone metabolism. J. Cell. Biochem. 99(3), 824–834 (2006). https://doi.org/10.1002/jcb.20915
S. Muruganandan, H.J. Dranse, J.L. Rourke, N.M. McMullen, C.J. Sinal, Chemerin neutralization blocks hematopoietic stem cell osteoclastogenesis. Stem Cells 31(10), 2172–2182 (2013). https://doi.org/10.1002/stem.1450
S. Muruganandan, A.A. Roman, C.J. Sinal, Role of chemerin/CMKLR1 signaling in adipogenesis and osteoblastogenesis of bone marrow stem cells. J. Bone Min. Res. 25(2), 222–234 (2010). https://doi.org/10.1359/jbmr.091106
J. Li, X. Chen, L. Lu, X. Yu, The relationship between bone marrow adipose tissue and bone metabolism in postmenopausal osteoporosis. Cytokine Growth Factor Rev. 52, 88–98 (2020). https://doi.org/10.1016/j.cytogfr.2020.02.003
B. Burguera, L.C. Hofbauer, T. Thomas, F. Gori, G.L. Evans, S. Khosla, B.L. Riggs, R.T. Turner, Leptin reduces ovariectomy-induced bone loss in rats. Endocrinology 142(8), 3546–3553 (2001). https://doi.org/10.1210/endo.142.8.8346
C.J. Li, Y. Xiao, M. Yang, T. Su, X. Sun, Q. Guo, Y. Huang, X.H. Luo, Long noncoding RNA Bmncr regulates mesenchymal stem cell fate during skeletal aging. J. Clin. Investig. 128(12), 5251–5266 (2018). https://doi.org/10.1172/JCI99044
Zhang, X., Robles, H., Magee, K.L., Lorenz, M.R., Wang, Z., Harris, C.A., Craft, C.S., Scheller, E.L.: A bone-specific adipogenesis pathway in fat-free mice defines key origins and adaptations of bone marrow adipocytes with age and disease. eLife. 10, (2021). https://doi.org/10.7554/eLife.66275
U.T. Iwaniec, R.T. Turner, Failure to generate bone marrow adipocytes does not protect mice from ovariectomy-induced osteopenia. Bone 53(1), 145–153 (2013). https://doi.org/10.1016/j.bone.2012.11.034
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82273294); the Science and Technology Department of Sichuan Province (2022YFS0136); the Chengdu Bureau of Science and Technology (2022-YF05-01316-SN); the National Natural Science Foundation of China (No.82204847); Key Research and Development Project of Science and Technology Department of Sichuan Province (2023YFS0332); and the 1.3.5 project for discipline of excellence, West China Hospital, Sichuan University (No. 2020HXFH008, No. ZYJC18003).
Author contributions
X.Y designed this research. J.L., L.L., L.L., C.W., Y.X. and L.T. were responsible for the experiments. Among them, J.L., L.L., L.L. and C.W. were in charge of the animal experiments, cellular experiments, and molecular experiments. Y.X. and L.T. were mainly responsible for the histopathological part. J.L., L.L., H.L. and X.Y. were responsible for the revision of the whole article. J.L. and L.L. contributed equally to this work. All authors contributed to the article and approved the submitted version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jiao Li, Lingyun Lu
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Lu, L., Liu, L. et al. The unique role of bone marrow adipose tissue in ovariectomy-induced bone loss in mice. Endocrine 83, 77–91 (2024). https://doi.org/10.1007/s12020-023-03504-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03504-6