Abstract
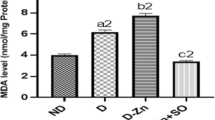
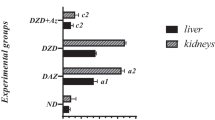
Diabetes mellitus is associated to a reduction of antioxidant defenses that leads to oxidative stress and complications in diabetic individuals. The present study was undertaken to investigate the effect of selenium on blood biochemical parameters, antioxidant enzyme activities, and tissue zinc levels in alloxan-induced diabetic rats fed a zinc-deficient diet. The rats were divided into two groups; the first group was fed a zinc-sufficient diet, while the second group was fed a zinc-deficient diet. Half of each group was treated orally with 0.5 mg/kg sodium selenite. Tissue and blood samples were taken from all animals after 28 days of treatment. At the end of the experiment, the body weight gain and food intake of the zinc-deficient diabetic animals were lower than that of zinc-adequate diabetic animals. Inadequate dietary zinc intake increased glucose, lipids, triglycerides, urea, and liver lipid peroxidation levels. In contrast, serum protein, reduced glutathione, plasma zinc and tissue levels were decreased. A zinc-deficient diet led also to an increase in serum glutamate oxaloacetate transaminase, glutamate pyruvate transaminase, and liver glutathione-S-transferase and to a decrease in serum alkaline phosphatase activity and glutathione peroxidase. Selenium treatment ameliorated all the values approximately to their normal levels. In conclusion, selenium supplementation presumably acting as an antioxidant led to an improvement of insulin activity, significantly reducing the severity of zinc deficiency in diabetes.




Similar content being viewed by others
References
Celik I, Yegin E, Odabasoglu F (2002) Effect of experimental diabetes mellitus on plasma lactate dehydrogenase and glutamic oxaloacetic transaminase levels in rabbits. Turkish J Biol 26:151–154
Nazıroğlu M, Dikici DM, Dursun S (2012) Role of oxidative stress and Ca2+ signaling on molecular pathways of neuropathic pain in diabetes: focus on TRP channels. Neurochem Res 37:2065–2075
Nazıroğlu M, Butterworth PJ (2005) Protective effects of moderate exercise with dietary vitamin C and E on blood antioxidative defense mechanism in rats with streptozotocin-induced diabetes. Can J Appl Physiol 30(2):172–185
Bonnefont-Rousselot D (2004) The role of antioxidant micronutrients in theprevention of diabetic complications. Treat Endocrinol 3:41–52
Nazıroğlu M, Cay M (2001) Protective role of intraperitoneally administered vitamin E and selenium on the antioxidative defense mechanisms in rats with diabetesinduced by streptozotocin. Biol Trace Elem Res 79:149–159
Simşek M, Naziroğlu M, Erdinç A (2005) Moderate exercise with a dietary vitamin Cand E combination protects against streptozotocin-induced oxidative damage to thekidney and lens in pregnant rats. ExpClinEndocrinol Diabetes 113:53–59
Bellomo EA, Meur G, Rutter GA (2011) Glucose regulates free cytosolic Zn2 concentration, Slc39 (ZiP), and metallothionein gene expression in primary pancreatic islet β-cells. Biol Chem 286:25778–25789
Ozcelik D, Nazıroglu M, Tunçdemir M, Celik O, Oztürk M, Flores-Arce MF (2012) Zinc supplementation attenuates metallothionein and oxidative stress changes in kidney of streptozotocin-induced diabetic rats. Biol Trace Elem Res 150:342–349
Nazıroğlu M (2009) Role of selenium on calcium signaling and oxidativestress-induced molecular pathways in epilepsy. Neurochem Res 34:2181–2191
Stapleton SR (2000) Selenium: insulin mimetic. Cell Mol Life Sci 57:1874–1879
Ezaki O (1990) The insulin-like effects of selenate in rat adipocytes. J BiolChem 265:1124–1128
Becker DJ, Reul B, Ozcelikay AT, Buchet JP, Henquin JC, Brichard SM (1996) Oral selenate improves glucose homeostasis and partly reverses abnormal expression of liver glycolytic and gluconeogenic enzymes in diabetic rats. Diabetologia 39:3–11
Pappas AC, Zoidis E, Georgiou CA, Demiris N, Surai PF, Fegeros K (2011) Influence oforganic selenium supplementation on the accumulation of toxic and essential trace elements involved in the antioxidant system of chicken. Food AdditContam Part A Chem Anal Control Expo Risk Assess 28:446–454
Kechrid Z, Derai EH, Layachi N (2007) The beneficial effect of vitamin E supplementation on zinc status, carbohydrate metabolism, transaminases and alkaline phosphatase activities in alloxan-diabetic rats fed on zinc deficiency diet. Int JDiabMetab 15:46–50
Southon S, Kechrid Z, Wright AJA, Fairweather-Tait S (1988) Effect of reduced dietary zinc intake on carbohydrate and zinc metabolism in the genetically diabetic mouse (C57BL/KsJdb+/db+). Brit Nutr 60(3):499–507
Tayibeh G, Mohammad N, Ebrahim I, Mohammad-Reza R (2011) Effect of vitamin E and selenium supplement on paraoxonase-1 activity, oxidized low density lipoprotein and antioxidant defense in diabetic rats. BioImpacts 1(2):121–128
Buege JA, Aust SD (1984) Microsomal lipid peroxidation. Methods Enzymol 105:302–310
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Jollow DJ, Mitchell JR, Zamppaglione Z, Gillette JR (1974) Bromobenzene induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolites. Pharmacology 11:151–159
Flohe L, Gunzler WA (1984) Analysis of glutathione peroxidase. Methods Enzymol 105:114–121
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione-S-transferase the first step in mercapturic acid formation. BiolChem 249(22):7130–7139
Bradford M (1976) A rapid and sensitive method for the quantities of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
McNall AD, Etherton TD, Fosmire GJ (1995) The impaired growth induced by zinc deficiency in rats is associated with decreased expression of the hepatic insulin like growth factor I and growth hormone receptor genes. Nutr 125(4):874–879
Nishi Y (1996) Zinc and growth. J Am Coll Nutr 15:340–344
Mueller AS, Bosse AC, Most E, Klomann SD, Schneider S, Pallauf J (2009) Regulation of the insulin antagonistic protein tyrosine phosphatase 1B by dietary Se studied in growing rats. J Nutr Biochem 20:235–247
Kechrid Z, Amamra S, Bouzerna N (2006) The effect of zinc deficiency on zinc status, carbohydrate metabolism and progesterone level in pregnant rats. J Turk Med Sci 36:337–342
Kechrid Z, Hamdi M, Nazıroğlu M, Flores-Arce M (2012) Vitamin D supplementation modulates blood and tissue zinc, liver glutathione and blood biochemical parameters in diabetic rats on a zinc deficient diet. Biol Trace Elem Res 148:371–377
Maret W (2003) Cellular zinc and redox states converge in the metallothionein/thionein pair. Nutr 133:1460s–1462s
Ghosh R, Mukherjee B, Chatterjee M (1994) A novel effect of selenium on streptozotocin-induced diabetic mice. Diabetes Res 25:165–171
Kechrid Z, Bouzerna N (2004) Effect of zinc deficiency and experimental diabetes on glutamate oxaloacetate, glutamate pyruvate aminotransferases and alkaline phosphatase activities in rats. Int J Diab Metab 11:14–18
Grefley S, Sandstead H (1993) Oxidation of alanine and B-hydroxybutyrate in late gestation by zinc restricted in rats. Nutr 113:1803–1810
Reiterer G, MacDonald R, Browning JD, Morrow J, Matveev SV, Daugherty A, Smart E, Toborek M, Hennig B (2005) Zinc deficiency increases plasma lipids and atherosclerotic markers in LDL-receptor-deficient mice. Nutr 135:2114–2118
Uchiyama S, Yamaguchi M (2003) Alteration in serum and bone component findings induced in streptozotocin-diabetic rats is restored by zinc acexamate. Int J Mol Med 12:949–954
Duzguner V, Kaya S (2007) Effect of zinc on the lipid peroxidation and the antioxidant defense systems of the alloxan-induced diabetic rabbits. Free Radic Biol Med 42:1481–1486
Espino J, Pariente JA, Rodríguez AB (2011) Role of melatonin on diabetes-related metabolic disorders. World J Diabetes 2(6):82–91
Yousef MI, El Hendy HA, El-Demerdash FM, Elagamy EI (2002) Dietary zinc deficiency induced-changes in the activity of enzymes and the levels of free radicals, lipids and protein electrophoretic behavior in growing rats. Toxicology 175:223–234
Rayman MP (2012) Selenium and human health. Lancet 379:1256–1268
Nazıroğlu M, Karaoğlu A, Aksoy AO (2004) Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology 195:221–230
Acknowledgment
ZK formulated the present hypothesis. ZK, MN, and MF were responsible for writing the report. WF was responsible for analysis of the data. This work was supported by research project no. FOI 120070054 funded by the Ministry of Higher Education, Algeria. There is no conflict interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Fatmi, W., Kechrid, Z., Nazıroğlu, M. et al. Selenium Supplementation Modulates Zinc Levels and Antioxidant Values in Blood and Tissues of Diabetic Rats Fed Zinc-Deficient Diet. Biol Trace Elem Res 152, 243–250 (2013). https://doi.org/10.1007/s12011-013-9613-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-013-9613-z