Abstract
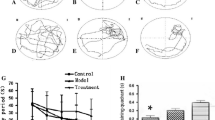
This study aimed to assess whether maifanite can improve the learning and memory, and antioxidant abilities of Alzheimer’s disease (AD) rats. The 70 rats were divided into seven groups: [A] normal control group, [B] AD model group, [C] sham group, [D] positive control group (donepezil), [E] low-dose maifanite group, [F] middle-dose maifanite group, [G] high-dose maifanite group. For [B], [D], [E], [F], and [G] groups, Aβ(25–35) ventricle injection was carried out, then respective medicine were administered once a day for 60 consecutive days. The step-down and step-through test were used to measure learning and memory ability. The hippocampus levels of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) were assayed. The hippocampus contents of Al, Fe, Cu, Zn, Se, and Mn were analyzed by inductively coupled plasma–atomic emission spectrometer. Maifanite decreased the acquisition errors and the retention errors while prolonging the step-down latency, and decreased the number of electric shocks while prolonging the first latency of AD rats. Aβ(25–35) ventricle injection initiated the decrease of SOD and GSH-Px activities and the increase of MDA content, and triggered the rise of Al, Fe, and Cu levels and the decline of Mn, Zn, and Se levels. The SOD and GSH-Px activities were enhanced followed by reduced MDA level, and the levels of Mn, Zn, and Se increased accompanied by Al, Fe, and Cu decreased in the maifanite treat groups. Maifanite could improve the learning and memory, and the antioxidant abilities of AD rats. Maifanite had the potential prevention and treatment for AD.
Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismutase
- GSH-Px:
-
Glutathione peroxidase
- ICP-AES:
-
Inductively coupled plasma–atomic emission spectrometer
References
Stéphan A, Phillips AG (2005) A case for a non-transgenic animal model of Alzheimer's disease. Genes Brain Behav 4:157–172
Castellani RJ, Rolston RK, Smith MA (2010) Alzheimer disease. Dis Mon 56:484–546
Ozcelik D, Uzun H, Nazıroglu M (2012) N-acetylcysteine attenuates copper overload-induced oxidative injury in brain of rat. Biol Trace Elem Res 147(1–3):292–298
Takahashi S, Takahashi I, Sato H, Kubota Y, Yoshida S, Muramatsu Y (2001) Age-related changes in the concentrations of major and trace elements in the brain of rats and mice. Biol Trace Elem Res 80(2):145–158
Nazıroglu M, Dikici DM, Dursun S (2012) Role of oxidative stress and Ca(2+) signaling on molecular pathways of neuropathic pain in diabetes: focus on TRP channels. Neurochem Res 37:2065–2075
Bonda DJ, Lee HG, Blair JA, Zhu X, Perry G, Smith MA (2011) Role of metal dyshomeostasis in Alzheimer's disease. Metallomics 3:267–270
Juan L, Zhang PY, Gao Y, Song XG, Dong JH (2008) Overview of maifanshi: its physico-chemical properties and nutritious function in drinking water. Environ Sci Technol 31:63–66
Zhang BG (2005) The progress of pharmaceutical research on the maifanite. Chinese Traditional Patent Med 27:1205–1208
Yao RZ (1991) The discussion on dissolution mechanism of trace elements in maifanite. Henan Geology 9:10–15
Liu ZY, Liu Q, Ma JK, Zhang CW (1986) Study on pharmacologic action of China maifanite. Jilin J Traditional Chinese Med 4:28–30
Nabeshima T, Nitta A (1994) Memory impairment and neuronal dysfunction induced by beta-amyloid protein in rats. Tohoku J Exp Med 174:241–249
Hashimoto M, Hossain S, Agdul H, Shidao O (2005) Docosahexaenoic acid-induced amelioration on impairment of memory learning in amyloid beta-infused rats relates to the decreases of amyloid beta and cholesterol levels in detergent-insoluble membrane fractions. Biochim Biophys Acta 1738:91–98
Diaz A, Limon D, Chávez R, Zenteno E, Guevara J (2012) Aβ25-35 injection into the temporal cortex induces chronic inflammation that contributes to neurodegeneration and spatial memory impairment in rats. J Alzheimers Dis 30:505–522
Huang TC, Lu KT, Wo YY, Wu YJ, Yang YL (2011) Resveratrol protects rats from Aβ-induced neurotoxicity by the reduction of iNOS expression and lipid peroxidation. PLoS One 6:e29102
Díaz A, De Jesús L, Mendieta L, Calvillo M, Espinosa B, Zenteno E, Guevara J, Limón ID (2010) The amyloid-beta25-35 injection into the CA1 region of the neonatal rat hippocampus impairs the long-term memory because of an increase of nitric oxide. Neurosci Lett 468:151–155
Limón ID, Díaz A, Mendieta L, Chamorro G, Espinosa B, Zenteno E, Guevara J (2009) Amyloid-beta(25–35) impairs memory and increases NO in the temporal cortex of rats. Neurosci Res 63:129–137
Paxinos G, Watson C (2005) The rat brain in sterotaxic coordinates, 5th edn. Elsevier, Sydney
Xu SY, Bian RL, Chen X (2002) Experimental protocols in pharmacology, 3rd edn. People’s Medical Publishing House, Beijing
Walton JR (2012) Aluminum disruption of calcium homeostasis and signal transduction resembles change that occurs in aging and Alzheimer's disease. J Alzheimers Dis 29:255–273
Carrí MT, Ferri A, Cozzolino M, Calabrese L, Rotilio G (2003) Neurodegeneration in amyotrophic lateral sclerosis: the role of oxidative stress and altered homeostasis of metals. Brain Res Bull 61:365–374
Dai XL, Sun YX, Jiang ZF (2006) Cu(II) potentiation of Alzheimer Abeta1-40 cytotoxicity and transition on its secondary structure. Acta Biochim Biophys Sin (Shanghai) 38:765–772
Huang X, Atwood CS, Hartshorn MA, Multhaup G, Goldstein LE, Scarpa RC, Cuajungco MP, Gray DN, Lim J, Moir RD, Tanzi RE, Bush AI (1999) The A beta peptide of Alzheimer's disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry 38:7609–7616
Chtourou Y, Trabelsi K, Fetoui H, Mkannez G, Kallel H, Zeghal N (2011) Manganese induces oxidative stress, redox state unbalance and disrupts membrane bound ATPases on murine neuroblastoma cells in vitro: protective role of silymarin. Neurochem Res 36:1546–1557
Mustak MS, Rao TS, Shanmugavelu P, Sundar NM, Menon RB, Rao RV, Rao KS (2008) Assessment of serum macro and trace element homeostasis and the complexity of inter-element relations in bipolar mood disorders. Clin Chim Acta 394:47–53
Lyubartseva G, Lovell MA (2012) A potential role for zinc alterations in the pathogenesis of Alzheimer's disease. Biofactors 38:98–106
Brewer GJ (2012) Copper excess, zinc deficiency, and cognition loss in Alzheimer's disease. Biofactors 38:107–113
Ishrat T, Parveen K, Khan MM, Khuwaja G, Khan MB, Yousuf S, Ahmad A, Shrivastav P, Islam F (2009) Selenium prevents cognitive decline and oxidative damage in rat model of streptozotocin-induced experimental dementia of Alzheimer's type. Brain Res 1281:117–127
Loef M, Schrauzer GN, Walach H (2011) Selenium and Alzheimer's disease: a systematic review. J Alzheimers Dis 26:81–104
Cardoso BR, Ong TP, Jacob-Filho W, Jaluul O, Freitas MI, Cozzolino SM (2010) Nutritional status of selenium in Alzheimer's disease patients. Br J Nutr 103:803–806
Acknowledgments
The authors thank the financial support of Natural Science Foundation of Guangxi Zhuang Autonomous Region, China (no. 2011GXNSFA018056).
Conflict of Interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, LF., Liao, HL., Huang, HM. et al. Potential Prevention and Treatment of Maifanite for Alzheimer's Disease Based on Behavior Test, Oxidative Stress Assay, and Trace Element Analysis in Hippocampus of Aβ(25–35)-Induced AD Rats. Biol Trace Elem Res 152, 50–56 (2013). https://doi.org/10.1007/s12011-012-9590-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9590-7