Abstract
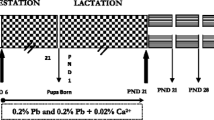
Oxidative stress and apoptosis facilitation in the developing central nervous system (CNS) have been inferred as two mechanisms related to lead’s neurotoxicity, and excessive reactive oxygen species (ROS) can promote oxidative stress and apoptosis facilitation. Few studies systematically investigated the potential relationship among oxidative stress, ROS generation, and apoptosis facilitation after lead exposure in earlier life as a whole. To better understand the adverse effect on the developing central nervous system (CNS) after lead exposure during pregnancy and lactation, the indexes of oxidative stress, apoptosis status, and Bax and Bcl-2 expression of offspring rats’ hippocampus were determined. Pregnant rats were randomly divided into four groups and given free access to drinking water which contained 0 %, 0.05 %, 0.1 %, and 0.2 % Pb(AC)2 respectively from gestation day 0 to postnatal day 21 (PND21). Results showed that ROS and malondialdehyde level of either PND7 or PND21 pups’ hippocampus were significantly raised; reduced glutathione level and superoxide dismutase activity were obviously decreased following the increase of blood and brain lead level. Similar to apoptotic indexes, Bax/Bcl-2 ratio increased after 0.1 % and 0.2 % Pb(AC)2 exposure, especially for the pups on PND7. Comparing with cortex, the hippocampus seemed much more sensitive to damage induced by lead. We concluded that the disruption of pro-oxidant and antioxidant balance and apoptosis facilitation could be associated with the mechanisms of neurotoxicity after lead exposure in earlier life.






Similar content being viewed by others
References
Lidsky TI, Schneider JS (2003) Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain 126:5–19
Sanders T, Liu Y, Buchner V, Paul B, Tchounwou PB (2009) Neurotoxic effects and biomarkers of lead exposure: a review. Rev Environ Health 24(1):15–45
Moreira EG, Vassilieff I, Vassilieff VS (2001) Developmental lead exposure: behaviour alterations in the short and long term. Neurotoxicol Teratol 23:489–495
Needleman HL, Schell A, Bellinger D, Leviton A, Allred EN (1990) The long-term effects of exposure to low doses of lead in childhood: an 11-year follow-up report. N Engl J Med 322(2):83–88
Shannon MW, Graef JW (1992) Lead intoxication in infancy. Pediatrics 89(1):87–90
Lockitch G (1993) Perspectives on lead toxicity. Clin Biochem 26(5):371–381
Rice DC (1993) Lead induced changes in learning: evidence for behavioral mechanisms from experimental animal studies. Neurotoxicology 14(2-3):167–178
West WL, Knight EM, Edwards CH, Manning M, Spurlock B, James H, Johnson AA, Oyemade UJ, Jackson Cole O, Westney LS (1994) Maternal low level lead and pregnancy outcomes. J Nutr 124:981S–986S
Goyer RA (1996) Results of lead research: prenatal exposure and neurological consequences. Environ Health Perspect 104:1050–1054
Meadows R (1996) Growing pains. Environ Health Perspect 104:146–149
Ruff HA, Markowitz ME, Bijur PE, Rosen JF (1996) Relationships among blood lead levels, iron deficiency, and cognitive development in two-year-old children. Environ Health Perspect 104:180–185
Moreira EG, Rosa GJ, Barros SB, Vassilieff VS, Vassillieff I (2001) Antioxidant defense in rat brain regions after developmental lead exposure. Toxicology 169(2):145–151
Jakubowski M (2011) Low-level environmental lead exposure and intellectual impairment in children—the current concepts of risk assessment. Int J Occup Med Environ Health 24(1):1–7
Rogan WJ, Ware JH, Rogan WJ, Ware JH (2003) Exposure to lead in children—how low is low enough? N Engl J Med 348(16):1515–1516
Jin Y, Liao Y, Lu C, Li G, Yu F, Zhi X, Xu J, Liu S, Liu M, Yang J (2006) Health effects in children aged 3–6 years induced by environmental lead exposure. Ecotoxicol Environ Saf 63:313–317
Adonaylo VN, Oteiza PI (1999) Lead intoxication: antioxidant defenses and oxidative damage in rat brain. Toxicology 135:77–85
Hermes-Lima M, Pereira B, Bechara EJ (1991) Are free radicals involved in lead poisoning? Xenobiotica 21(8):1085–1090
Monteiro HP, Abdalla DS, Arcuri AS, Bechara EJ (1985) Oxygen toxicity related to exposure to lead. Clin Chem 31(10):1673–1686
Bondy SC (1992) Reactive oxygen species: relation to aging and neurotoxic damage. Neurotoxicology 13(1):87–100
Bussche JV, Soares EV (2011) Lead induces oxidative stress and phenotypic markers of apoptosis in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 90(2):679–687
Oberto A, Marks N, Evans HL, Guidotti A (1996) Lead (Pb+2) promotes apoptosis in newborn rat cerebellar neurons: pathological implications. J Pharmacol Exp Ther 279(1):435–442
Chu J, Tong M, de la Monte SM (2007) Chronic ethanol exposure causes mitochondrial dysfunction and oxidative stress in immature central nervous system neurons. Acta Neuropathol 113(6):659–673
Cory S, Adams JM (2002) The Bcl-2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2:647–656
Roth KA, D’Sa C (2001) Apoptosis and brain development. Ment Retard Dev Disabil Res Rev 7(4):261–266
Mooney SM, Miller MW (2000) Expression of Bcl-2, Bax, and caspase-3 in the brain of the developing rat. Dev Brain Res 123:103–117
Clark RS, Kochanek PM, Adelson PD, Bell MJ, Carcillo JA, Chen M, Wisniewski SR, Janesko K, Whalen MJ, Graham SH (2000) Increases in bcl-2 protein in cerebrospinal fluid and evidence for programmed cell death in infants and children after severe traumatic brain injury. J Pediatr 137(2):197–204
Petit TL, Alfano DP, LeBoutillier JC (1983) Early lead exposure and the hippocampus: a review and recent advances. Neurotoxicology 4(1):79–94
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Brescia F, Sarti M (2008) Modification to the Lampariello approach to evaluate reactive oxygen species production by flow cytometry. Cytom A 73:175–179
Lockitch G (1993) Blood lead levels in children. CMAJ 149(2):139–142
Li RG, Li TT, Hao L, Xu X, Na J (2009) Hydrogen peroxide reduces lead-induced oxidative stress to mouse brain and liver. Bull Environ Contam Toxicol 82(4):419–422
Fernández-Novoa L, Alvarez XA, Sempere JM, Miguel-Hidalgo JJ, Díaz J, Franco-Maside A, Cacabelos R (1997) Effects of anapsos on the activity of the enzyme Cu–Zn-superoxide dismutase in an animal model of neuronal degeneration. Methods Find Exp Clin Pharmacol 19(2):99–106
Shearer J, Neupane KP, Callan PE (2009) Metallopeptide based mimics with substituted histidines approximate a key hydrogen bonding network in the metalloenzyme nickel superoxide dismutase. Inorg Chem 48(22):10560–10571
Pulido MD, Parrish AR (2003) Metal-induced apoptosis: mechanisms. Mutat Res 533(1–2):227–241
Han JM, Chang BJ, Li TZ, Choe NH, Quan FS, Jang BJ, Cho IH, Hong HN, Lee JH (2007) Protective effects of ascorbic acid against lead-induced apoptotic neurodegeneration in the developing rat hippocampus in vivo. Brain Res 1185:68–74
Chao SL, Moss JM, Harry GJ (2007) Lead-induced alterations of apoptosis and neurotrophic factor mRNA in the developing rat cortex, hippocampus and cerebellum. J Biochem Mol Toxicol 21(5):265–272
Ferrer I, Serrano T, Soriano E (1990) Naturally occurring cell death in the subicular complex and hippocampus in the rat during development. Neurosci Res 8(1):60–66
Spreafico R, Frassoni C, Arcelli P, Selvaggio M, De Biasi S (1995) In situ labeling of apoptotic cell death in the cerebral cortex and thalamus of rats during development. J Comp Neurol 363(2):281–295
Sharifi AM, Mousavi SH, Jorjani M (2010) Effect of chronic lead exposure on pro-apoptotic Bax and anti-apoptotic Bcl-2 protein expression in rat hippocampus in vivo. Cell Mol Neurobiol 30(5):769–774
Reed JC (1997) Bcl-2 family proteins and the hormonal control of cell life and death in normalcy and neoplasia. Vitam Horm 53:99–138
Yang E, Korsmeyer SJ (1996) Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood 88(2):386–401
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74(4):609–619
Zha H, Fisk HA, Yaffe MP, Mahajan N, Herman B, Reed JC (1996) Structure–function comparisons of the proapoptotic protein Bax in yeast and mammalian cells. Mol Cell Biol 16(11):6494–6508
Adrain C, Martin SJ (2001) The mitochondrial apoptosome: a killer unleashed by the cytochrome C. Trends Biochem Sci 26(6):390–397
Ercal N, Luo X, Matthews RH, Armstrong DW (1996) In vitro study of the metabolic effects of D-amino acids. Chirality 8(1):24–29
James D, Parone PA, Terradillos O, Lucken-Ardjomande S, Montessuit S, Martinou JC (2007) Mechanisms of mitochondrial outer membrane permeabilization. Novartis Found Symp 287:170–182
Acknowledgments
We thank Mrs. Beili Li (Center laboratory, School of Public Health, China Medical University) for her excellent work in lead determination using atomic absorption spectrophotometry. This study was supported by Provincial Education Foundation of Liaoning (No. L2010703).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, X., Jin, C., Yang, J. et al. Prenatal and Lactational Lead Exposure Enhanced Oxidative Stress and Altered Apoptosis Status in Offspring Rats’ Hippocampus. Biol Trace Elem Res 151, 75–84 (2013). https://doi.org/10.1007/s12011-012-9531-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9531-5