Abstract
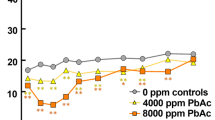
Despite reduction in environmental lead, chronic lead exposure still possess a public health hazard, particularly in children, with devastating effects on developing CNS. To investigate the mechanism of this neurotoxicity, young and adult rats were used to study whether exposure to 500 ppm concentrations of lead could induce apoptosis in hippocampus. 2–4 and 12–14-week-old rats received lead acetate in concentration of 500 ppm for 40 days. Control animals received deionized distilled water. In lead-treated groups, the blood lead levels were increased by 3–4 folds. Light and electron microscopical study of hippocampus revealed increased apoptotic cells. Western blot analysis of Bax and Bcl-2 (pro- and anti-apoptotic gene products, respectively) indicated higher expression of Bax protein and no significant change in bcl-2 expression and accordingly increased the Bax/Bcl-2 ratio compared to control group, confirming the histological study. In conclusion, these data suggest that neurotoxicity of chronic lead exposure in hippocampus in vivo may partly be due to facilitation of apoptosis.


Similar content being viewed by others
References
Bellinger D, Sloman J, Leviton A, Rabinowitz M, Needleman HL, Waternaux C (1991) Low-level lead exposure and children’s cognitive function in preschool years. Pediatrics 87:219–227
Bellinger D, Stiles K, Needleman HL (1992) Low-level lead exposure intelligence and academic achievement. A long-term follow-up study. Pediatrics 89:855–861
Bleecker ML, Ford DP, Lindgren KN, Hoese VM, Walsh KS, Vaughan CG (2005) Differential effects of lead exposure on components of verbal memory. Occup Environ Med 62:181–187
Boise LH, Gonzales-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunes G, Thompson CB (1993) Bcl-x, a Bcl-2- related gene that functions as a dominant regulator of apoptotic cell death. Cell 74:597–608
Boyd JM, Gallo GJ, Elangovan B, Houghton AB, Malstrom S, Avery BJ, Ebb RG, Subramanian T, Chittenden T, Lutz RJ, Chinnadurai G (1995) Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene 11:1921–1928
Canfield RL, Gendle MH, Cory-Slechta DA (2004) Impaired neuropsychological functioning in lead-exposed children. Dev Neuropsychol 26:513–540
Chao SL, Moss JM, Harry GJ (2007) Lead-induced alterations of apoptosis and neurotrophic factor mRNA in the developing rat cortex, hippocampus, and cerebellum. J Biochem Mol Toxicol 21:265–272
Chen J, Zhu W, Chen Q, Lu L (2004) Effects of lead on the Brn-3a expression and the apoptosis in hippocampus neurons. Wei Sheng Yan Jiu 33:134–136
Chittenden T, Harrington EA, O’Connor R, Flemington C, Lutz RJ, Evan GI, Guild BC (1995) Induction of apoptosis by the Bcl-2 homologue Bak. Nature 374:733–736
Choi DW (1988) Glutamate neurotoxicity, diseases of the nervous system. Neuron 1:623–634
Franclin JL, Johnson EM Jr (1992) Suppression of programmed cell death by sustained elevation of cytoplasmic calcium. Trends Neurosci 15:501–508
Goldstein GW (1993) Evidence that lead acts as a calcium substitute in second messenger metabolism. Neurotixicology 14:97–101
Goldstein GW (1994) Brain capillaries: a target for inorganic lead poisoning. Neurotoxicology 5:167–176
Han JM, Chang BJ, Li TZ, Choe NH, Quan FS, Jang BJ, Cho IH, Hong HN, Lee JH (2007) Protective effects of ascorbic acid against lead-induced apoptotic neurodegeneration in the developing rat hippocampus in vivo. Brain Res 14:68–74
Hassouna I, Wickert H, Zimmerman M, Gillardon F (1996) Increase in bax expression in substantia nigra following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment of mice. Neurosci Lett 204:85–88
He L, Poblenz AT, Medrano CJ, Fox DA (2000a) Lead and calcium produce rod photoreceptor cell apoptosis by opening the mitochondrial permeability transition pore. J Biol Chem 275:12175–12184
He L, Poblenz AT, Medrano CJ, Fox DA (2000b) Lead and calcium produce rod photoreceptor cell apoptosis by opening the mitochondrial permeability transition pore. J Biol Chem 275:12175–12184
He L, Perkins GA, Poblenz AT, Harris JB, Hung M, Ellisman MH, Fox DA (2003) Bcl-xL overexpression blocks baxmediated mitochondrial contact site formation and apoptosis in rod photoreceptors of lead-exposed mice. Proc Natl Acad Sci USA 100:1022–1027
Hockenbery DM, Nunes G, Milliman C, Schreiber RD, Korsmeyer SJ (1990) Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 384:334–336
Kiran Kumar B, Prabhakara Rao Y, Noble T, Weddington K, McDowell VP, Rajanna S, Bettaiya R (2009) Lead-induced alteration of apoptotic proteins in different regions of adult rat brain. Toxicol Lett 10:56–60
Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW (1993) MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to Bcl-2. Proc Natl Acad Sci USA 90:3516–3520
Lansdown R, Yule W, Urbanowicz MA, Hunter J (1986) The relationship between blood-lead concentrations, intelligence, attainment and behaviour in a school population: the second London study. Int Arch Occup Environ Health 57:225–235
Lidsky TI, Schneider JS (2003) Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain 126:5–19
Lin EY, Orlofsky A, Berger MS, Prystowsky MB (1993) Characterization of A1, a novel hemopoietic-specific early-response gene with sequence similarity to bcl-2. J Immunol 151:1979–1988
Marchetti C (2003) Molecular targets of lead in brain neurotoxicity. Neurotox Res 5:221–236
Niu Y, Zhang R, Cheng Y, Sun X, Tian J (2002) Effect of lead acetate on the apoptosis and the expression of bcl-2 and bax genes in rat brain cells. Zhonghua Yu Fang Yi Xue Za Zhi 36:30–33
Oberto A, Marks N, Evans HL, Guidotti A (1996) Lead promotes apoptosis in newborn rat cerebellar neurons: pathological implications. J Pharmacol Exp Ther 279:435–442
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimers in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619
Petit TL, Alfano DP, LeBoutillier JC (1983) Early lead exposure and the hippocampus: a review and recent advances. Neurotoxicology 4:79–94
Reed JC (1997) Bcl-2 family proteins and the control of cell life and death in normalcy and neoplasia. Vitam Horm 53:99–138
Schwartz J, Otto D (1997) Blood lead hearing thresholds and neurobehavioral development in children and youth. Arch Environ Health 42:153–160
Sharifi AM, Mousavi SH (2008) Study of lead-induced toxicity in PC12 cells: role of apoptosis. Toxicol Mech Methods 18:1–5
Sharifi AM, Baniasadi S, Jorjani M, Rahimi F, Bakhshayesh M (2002) Investigation of acute lead poisoning on apoptosis in hippocampus invivo. Neurosci Lett 329:45–48
Takayama S, Sato T, Krajewski S, Kochel K, Irie S, Millan JA, Reed JC (1995) Cloning and functional analysis of BAG-1: a novel Bcl-2-binding protein with anti-cell death activity. Cell 80:279–284
Tavakoli-Nezhad M, Barron AJ, Pitts DK (2001) Postnatal inorganic lead exposure decreases the number of spontaneously active midbrain dopamine neurons in the rat. Neurotoxicology 22:259–269
Winneke G, Kramer U (1997) Neurobehavioral aspects of lead neurotoxicity in children. Eur J Pub Health 5:65–69
Winneke G, Kramer U, Brockhaus A, Ewers U, Kujanek G, Lechner H, Janke W (1983) Neuropsychological studies in children with elevated tooth-lead concentrations. Arch Occup Environ Health 51:231–252
Wyllie AH (1981) Cell death: a new classification separating apoptosis from necrosis. In: Brown ID, Lockshin RA (eds) Cell death in biology and pathology. Chapman and Hall, New York, pp 9–34
Xiang J, Chao DT, Korsmeyer SJ (1996) BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases. Proc Natl Acad Sci USA 10:14559–14563
Yang E, Korsmeyer J (1996) Molecular thanatopsis: a discourse on the bcl-2 family and cell death. Blood 88:336–401
Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ (1995) Bad, a heterodimeric partner for Bcl-xl and Bcl-2 displace Bax and promotes cell death. Cell 80:285–291
Yin C, Knudson CM, Korsmeyer SJ, Van Dyke T (1997) Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature 13:637–640
Zha H, Aime-Sempe C, Takaaki S, Reed JC (1996) Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J Biol Chem 29:7440–7444
Zhang J, Wang XF, Lu ZB, Liu NQ, Zhao BL (2004) The effects of meso-2,3-dimercaptosuccinic acid and oligomeric procyanidins on acute lead neurotoxicity in rat hippocampus. Free Radic Biol Med 37:1037–1050
Acknowledgment
This work was supported by a grant from the research section of Iran University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharifi, A.M., Mousavi, S.H. & Jorjani, M. Effect of Chronic Lead Exposure on Pro-Apoptotic Bax and Anti-Apoptotic Bcl-2 Protein Expression in Rat Hippocampus In Vivo. Cell Mol Neurobiol 30, 769–774 (2010). https://doi.org/10.1007/s10571-010-9504-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-010-9504-1