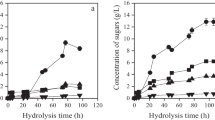
Abstract
The complex structure of rice straw is such that its bioconversion requires multiple physical and chemical pretreatment steps. In this study, it was found that a large amount of soluble polysaccharides (SPs) are formed during the pretreatment of straw. The yield of NaOH-based SPs (4.8%) was much larger than that of ball-milled SPs (1.5%) and H2SO4-based SPs (1.1%). For all the pretreatments, the ratio of phenolic compounds to saccharides (P/S) for each type of SPs increased upon increasing the concentration of ethanol in the order of 90% > 70% > 50%. The yield of NaOH-based SPs was much higher than that of acid-based and ball-milled SPs. The changes in the 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS), ferric reducing antioxidant power assay (FRAP), and 2,2-diphenyl-1-picrylhydrazyl (DPPH) of SPs follow the same rule, i.e., the higher the P/S ratio, the higher the antioxidant values of the SPs. The flow cytometry and laser scanning microscopy results show that the P/S ratio can significantly influence the effect of SPs on microbial growth and cell membrane permeability. Upon varying the ethanol concentration in the range of 50–90%, the P/S ratio increased from 0.02 to 0.17, resulting in an increase in the promoting effects of the SPs on yeast cell growth. Furthermore, H2O2, NAD+/NADH, and NADP+/NADPH assays indicate that SPs with a high P/S ratio can reduce intracellular H2O2 and change the intracellular redox status.







Similar content being viewed by others
Data Availability
No data was used for the research described in the article.
References
Sakai, K., Hassan, M. A., Vairappan, C. S. and Shirai, Y. (2022) Promotion of a green economy with the palm oil industry for biodiversity conservation: A touchstone toward a sustainable bioindustry. Journal of Bioscience and Bioengineering.
Favaro, L., Jansen, T., & van Zyl, W. H. (2019). Exploring industrial and natural Saccharomyces cerevisiae strains for the bio-based economy from biomass: The case of bioethanol. Critical reviews in biotechnology, 39, 800–816.
Zhang, F., Bunterngsook, B., Li, J.-X., Zhao, X.-Q., Champreda, V., Liu, C.-G. and Bai, F.-W. (2019), in Advances in bioenergy, vol. 4, Elsevier, pp. 79–119.
Zheng, W., Zheng, Q., Xue, Y., Hu, J., & Gao, M.-T. (2017). Influence of rice straw polyphenols on cellulase production by Trichoderma reesei. Journal of bioscience and bioengineering, 123, 731–738.
Sharma, J., Kumar, S. S., Kumar, V., Malyan, S. K., Mathimani, T., Bishnoi, N. R., & Pugazhendhi, A. (2020). Upgrading of microalgal consortia with CO2 from fermentation of wheat straw for the phycoremediation of domestic wastewater. Bioresource technology, 305, 123063.
Chen, X., Wang, X., Xue, Y., Zhang, T.-A., Li, Y., Hu, J., Tsang, Y. F., Zhang, H., & Gao, M.-T. (2018). Influence of rice straw-derived dissolved organic matter on lactic acid fermentation by Rhizopus oryzae. Journal of bioscience and bioengineering, 125, 703–709.
Zheng, W., Chen, X., Xue, Y., Hu, J., Gao, M.-T., & Tsang, Y. F. (2017). The influence of soluble polysaccharides derived from rice straw upon cellulase production by Trichoderma reesei. Process Biochemistry, 61, 130–136.
Liu, J., Luo, Y., Guo, T., Tang, C., Chai, X., Zhao, W., Bai, J., & Lin, Q. (2020). Cost-effective pigment production by Monascus purpureus using rice straw hydrolysate as substrate in submerged fermentation. Journal of bioscience and bioengineering, 129, 229–236.
Chen, X., Wang, X., Xue, Y., Zhang, T.-A., Hu, J., Tsang, Y. F., & Gao, M.-T. (2018). Tapping the bioactivity potential of residual stream from its pretreatments may be a green strategy for low-cost bioconversion of rice straw. Applied Biochemistry and Biotechnology, 186, 507–524.
Wang, X., Cui, S., Hu, J., Ma, X., Zhang, T.-A., Tsang, Y. F., Li, J., & Gao, M.-T. (2019). Saccharides in straw hydrolysate decrease cell membrane damage by phenolics by inducing the formation of extracellular matrix in yeast. Carbohydrate Polymers, 219, 414–422.
Velmurugan, P., Hur, H., Balachandar, V., Kamala-Kannan, S., Lee, K.-J., Lee, S.-M., Chae, J.-C., Shea, P. J., & Oh, B.-T. (2011). Monascus pigment production by solid-state fermentation with corn cob substrate. Journal of bioscience and bioengineering, 112, 590–594.
Mitchell, K. F., Zarnowski, R. and Andes, D. R. (2016) The extracellular matrix of fungal biofilms. Fungal biofilms and related infections, 21–35.
Zara, G., Zara, S., Pinna, C., Marceddu, S., & Budroni, M. (2009). FLO11 gene length and transcriptional level affect biofilm-forming ability of wild flor strains of Saccharomyces cerevisiae. Microbiology, 155, 3838–3846.
Beauvais, A., Loussert, C., Prevost, M. C., Verstrepen, K., & Latgé, J. P. (2009). Characterization of a biofilm-like extracellular matrix in FLO1-expressing Saccharomyces cerevisiae cells. FEMS yeast research, 9, 411–419.
Liu, H.-M., Wang, F.-Y., & Liu, Y.-L. (2016). Hot-compressed water extraction of polysaccharides from soy hulls. Food Chemistry, 202, 104–109.
Xue, Y., Wang, X., Chen, X., Hu, J., Gao, M.-T., & Li, J. (2017). Effects of different cellulases on the release of phenolic acids from rice straw during saccharification. Bioresource Technology, 234, 208–216.
Wang, X., Tsang, Y. F., Li, Y., Ma, X., Cui, S., Zhang, T.-A., Hu, J., & Gao, M.-T. (2017). Inhibitory effects of phenolic compounds of rice straw formed by saccharification during ethanol fermentation by Pichia stipitis. Bioresource Technology, 244, 1059–1067.
Richmond, G. (2002). Molecular bonding and interactions at aqueous surfaces as probed by vibrational sum frequency spectroscopy. Chemical reviews, 102, 2693–2724.
Wang, Y. Z., Yang, J., Wei, H., Hou, R., Shi, J., Jin, Z., Yang, F., Hu, J., & Gao, M.-T. (2020). Reduction of fermentation-associated stresses by straw-based soluble saccharides for enhancing ethanol production. Journal of Agricultural and Food Chemistry, 68, 5863–5872.
Duan, Y., Ge, C., Liu, S., Chen, C., Zhou, M. J. P. B. and Physiology. (2013) Effect of phenylpyrrole fungicide fludioxonil on morphological and physiological characteristics of Sclerotinia sclerotiorum. 106, 61-67
Singleton, V. L., & Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American journal of Enology and Viticulture, 16, 144–158.
Hou, R., Hu, J., Wang, Y., Wei, H., & Gao, M.-T. (2020). Simultaneous production of cellulase and ferulic acid esterase by Penicillium decumbens with rice straw as the sole carbon source. Journal of bioscience and bioengineering, 129, 276–283.
Belwal, T., Dhyani, P., Bhatt, I. D., Rawal, R. S., & Pande, V. (2016). Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food chemistry, 207, 115–124.
Hu, H., Zhang, Z., Lei, Z., Yang, Y., & Sugiura, N. (2009). Comparative study of antioxidant activity and antiproliferative effect of hot water and ethanol extracts from the mushroom Inonotus obliquus. Journal of bioscience and bioengineering, 107, 42–48.
Wang, X., Cui, S., Hu, J., Ma, X., Zhang, T.-A., Tsang, Y. F., Li, J. and Gao, M.-T. J. C. p. (2019) Saccharides in straw hydrolysate decrease cell membrane damage by phenolics by inducing the formation of extracellular matrix in yeast. 219, 414–422.
Park, E. Y., Saito, T., Dojima, T., Horiba, M., Toriyama, M., & Okabe, M. (1999). Visualization of a recombinant gene protein in the baculovirus expression vector system using confocal scanning laser microscopy. Journal of bioscience and bioengineering, 87, 756–761.
Sun, Y., Hou, S., Song, S., Zhang, B., Ai, C., Chen, X. and Liu, N. J. I. j. o. b. m. (2018) Impact of acidic, water and alkaline extraction on structural features, antioxidant activities of Laminaria japonica polysaccharides. 112, 985–995.
Bello, B., Mustafa, S., Tan, J. S., Ibrahim, T. A. T., Tam, Y. J., Ariff, A. B., Manap, M. Y., & Abbasiliasi, S. J. B. (2018). Evaluation of the effect of soluble polysaccharides of palm kernel cake as a potential prebiotic on the growth of probiotics, 8, 1–14.
Lahaye, M., & Robic, A. (2007). Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules, 8, 1765–1774.
Peasura, N., Laohakunjit, N., Kerdchoechuen, O., & Wanlapa, S. (2015). Characteristics and antioxidant of Ulva intestinalis sulphated polysaccharides extracted with different solvents. International journal of biological macromolecules, 81, 912–919.
Ballesteros, L. F., Cerqueira, M. A., Teixeira, J. A. and Mussatto, S. I. J. C. P. (2015) Characterization of polysaccharides extracted from spent coffee grounds by alkali pretreatment. 127, 347–354.
Li, A.-L., Hou, X.-D., Lin, K.-P., Zhang, X., & Fu, M.-H. (2018). Rice straw pretreatment using deep eutectic solvents with different constituents molar ratios: Biomass fractionation, polysaccharides enzymatic digestion and solvent reuse. Journal of bioscience and bioengineering, 126, 346–354.
Ahmad, A., Kim, S.-H., Ali, M., Park, I., Kim, J.-S., Kim, E.-H., Lim, J.-J., Kim, S.-K., & Chung, I.-M. (2013). New chemical constituents from Oryza sativa straw and their algicidal activities against blue-green algae. Journal of agricultural and food chemistry, 61, 8039–8048.
Yang, F., Jin, Z., Nawaz, M., Xiao, Y., Jiang, Y., Hu, J., Li, J., & Gao, M.-T. (2022). Oligosaccharides in straw hydrolysate could improve the production of single-cell protein with Saccharomyces cerevisiae. Journal of the Science of Food and Agriculture, 102, 2928–2936.
Wang, Y. Z., Zheng, J., Nawaz, M., Yang, F., Hu, J., & Gao, M.-T. (2022). Oligosaccharide-phenolic compound conjugates in soluble polysaccharides from rice straw alleviate ethanol fermentation stresses in Saccharomyces cerevisiae. Industrial Crops and Products, 181, 114782.
Bandikari, R., Poondla, V., & Obulam, V. S. R. J. B. (2014). Enhanced production of xylanase by solid state fermentation using Trichoderma koeningi isolate. Effect of pretreated agro-residues., 4, 655–664.
Kallel, F., Driss, D., Bouaziz, F., Belghith, L., Zouari-Ellouzi, S., Haddar, A., Chaabouni, S. E. and Ghorbel, R. J. R. A. (2015) Polysaccharide from garlic straw: Extraction, structural data, biological properties and application to beef meat preservation. 5, 6728–6741.
Chen, X., Xue, Y., Hu, J., Tsang, Y. F., & Gao, M.-T. (2017). Release of polyphenols is the major factor influencing the bioconversion of rice straw to lactic acid. Applied biochemistry and biotechnology, 183, 685–698.
Zhu Wang, Y., Zheng, J., Nawaz, M., Yang, F., Hu, J., & Gao, M.-T. (2022). Oligosaccharide-phenolic compound conjugates in soluble polysaccharides from rice straw alleviate ethanol fermentation stresses in Saccharomyces cerevisiae. Industrial Crops and Products, 181, 114782.
Yan, J.-K., Wang, C., Yu, Y.-B., Wu, L.-X., Chen, T.-T., & Wang, Z.-W. (2021). Physicochemical characteristics and in vitro biological activities of polysaccharides derived from raw garlic (Allium sativum L) bulbs via three-phase partitioning combined with gradient ethanol precipitation method. Food Chemistry, 339, 128081.
Xu, J., Yue, R.-Q., Liu, J., Ho, H.-M., Yi, T., Chen, H.-B., & Han, Q.-B. (2014). Structural diversity requires individual optimization of ethanol concentration in polysaccharide precipitation. International journal of biological macromolecules, 67, 205–209.
Zhang, K., Yuan, D., Li, C., & Fu, X. (2021). Physicochemical properties and bioactivity of polysaccharides from Sargassum pallidum by fractional ethanol precipitation. International Journal of Food Science & Technology, 56, 3536–3545.
Xing, S., Zhang, X., Ke, H., Lin, J., Huang, Y. and Wei, G. J. C. C. J. (2018) Physicochemical properties of polysaccharides from Dendrobium officinale by fractional precipitation and their preliminary antioxidant and anti-HepG2 cells activities in vitro. 12, 1–10.
Verma, B., Hucl, P., & Chibbar, R. J. F. C. (2009). Phenolic acid composition and antioxidant capacity of acid and alkali hydrolysed wheat bran fractions., 116, 947–954.
Yang, X., Huang, M., Qin, C., Lv, B., Mao, Q., & Liu, Z. (2017). Structural characterization and evaluation of the antioxidant activities of polysaccharides extracted from Qingzhuan brick tea. International Journal of Biological Macromolecules, 101, 768–775.
Akpinar, Ö., Usal, G. J. F. and Processing, B. (2015) Investigation of the effect of temperature and alkaline concentration on the solubilization of phenolic acids from dilute acid-pretreated wheat straw. 95, 272-280
Wang, Y. Z., Yang, J., Wei, H., Hou, R., Shi, J., Jin, Z., Yang, F., Hu, J., Gao, M.-T. J. J. o. a. and chemistry, f. (2020) Reduction of fermentation-associated stresses by straw-based soluble saccharides for enhancing ethanol production. 68, 5863–5872.
Mah, T.-F. C. and O'Toole, G. A. J. T. i. m. (2001) Mechanisms of biofilm resistance to antimicrobial agents. 9, 34–39.
Auesukaree, C. (2017). Molecular mechanisms of the yeast adaptive response and tolerance to stresses encountered during ethanol fermentation. Journal of bioscience and bioengineering, 124, 133–142.
Mitchell, K. F., Zarnowski, R., Andes, D. R. J. F. b. and infections, r. (2016) The extracellular matrix of fungal biofilms. 21–35.
Siddiqi, M. K., Alam, P., Chaturvedi, S. K., Nusrat, S., Shahein, Y. E. and Khan, R. H. J. I. j. o. b. m. (2017) Attenuation of amyloid fibrillation in presence of Warfarin: A biophysical investigation. 95, 713–718.
Kim, J., Kwon, J., Kim, M., Do, J., Lee, D. and Han, H. J. P. J. (2016) Low-dielectric-constant polyimide aerogel composite films with low water uptake. 48, 829-834
Wei, H., Wang, Y., Jin, Z., Yang, F., Hu, J., Gao, M.-T. J. J. o. b. and bioengineering. (2021) Utilization of straw-based phenolic acids as a biofugicide for a green agricultural production. 131, 53–60.
Gu, H., Zhang, J., Bao, J. J. B. and bioengineering. (2015) High tolerance and physiological mechanism of Zymomonas mobilis to phenolic inhibitors in ethanol fermentation of corncob residue. 112, 1770-1782
Mendes, V., Vilaça, R., de Freitas, V., Ferreira, P. M., Mateus, N., Costa, V. J. O. M. and longevity, C. (2015) Effect of myricetin, pyrogallol, and phloroglucinol on yeast resistance to oxidative stress. 2015.
Hasanuzzaman, M., Banerjee, A., Bhuyan, M. B., Roychoudhury, A., Al Mahmud, J. and Fujita, M. J. P. (2019) Targeting glycinebetaine for abiotic stress tolerance in crop plants: physiological mechanism, molecular interaction and signaling. 88, 185.
Acknowledgements
The authors appreciate the support of the National Key R&D Program of China (SQ2021YFE010400) and the National Natural Science Foundation of China (32071640, 51878646).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nawaz, M., Jiang, Y., Xiao, Y. et al. Influence of Different Pretreatment Steps on the Ratio of Phenolic Compounds to Saccharides in Soluble Polysaccharides Derived from Rice Straw and Their Effect on Ethanol Fermentation. Appl Biochem Biotechnol 195, 4552–4569 (2023). https://doi.org/10.1007/s12010-023-04337-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04337-9