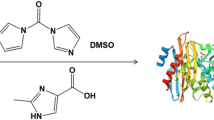
Abstract
Metal–organic frameworks (MOFs) can be used as the immobilization carriers to protect the physicochemical properties of enzymes and improve their catalytic performance. Herein, we report an in situ co-precipitation method to immobilize lipase from Candida sp. 99–125 in Cu-BTC MOF (BTC = 1, 3, 5-benzene tricarboxylic acid, H3BTC). Characterizations of the immobilized lipase (lipase@Cu-BTC) have confirmed the entrapment of lipase molecules in Cu-BTC MOF. The immobilized lipase has been successfully applied for resolving N-hydroxymethyl vince lactam (N-HMVL) and its catalytic activity is five times that of native enzyme. More importantly, we found that Cu-BTC MOF can afford powerful protection for enzyme in nearly dry organic solvent and endow the immobilized lipase with excellent reusability and storage stability. Our present study may widen the application of immobilized enzyme with MOF as the immobilized carrier.
Graphical Abstract









Similar content being viewed by others
Data availability
Data and materials made available on reasonable request.
References
Ghanem, A., & Aboul-Enein, H. Y. (2005). Application of lipases in kinetic resolution of racemates. Chirality, 17(1), 1–15. https://doi.org/10.1002/chir.20089
Fang, Y., Huang, X. J., Chen, P. C., & Xu, Z. K. (2011). Polymer materials for enzyme immobilization and their application in bioreactors. BMB Reports, 44(2), 87–95. https://doi.org/10.5483/BMBRep.2011.44.2.87
Sheldon, R. A. (2007). Enzyme immobilization: The quest for optimum performance. Advanced Synthesis & Catalysis, 349(8–9), 1289–1307. https://doi.org/10.1002/adsc.200700082
Mohamad, N. R., Marzuki, N. H., Buang, N. A., Huyop, F., & Wahab, R. A. (2021). An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnology & Biotechnological Eauipment, 29(2), 205–220. https://doi.org/10.1080/13102818.2015.1008192
Bhushan, I., Parshad, R., Qazi, G. N., Ingavle, G., Rajan, C. R., Ponrathnam, S., & Gupta, V. K. (2008). Lipase enzyme immobilization on synthetic beaded macroporous copolymers for kinetic resolution of chiral drugs intermediates. Process Biochemistry, 43(4), 321–330. https://doi.org/10.1016/j.procbio.2007.11.019
Xun, E. N., Lv, X. L., Kang, W., Wang, J. X., Zhang, H., Wang, L., & Wang, Z. (2012). Immobilization of Pseudomonas fluorescens lipase onto magnetic nanoparticles for resolution of 2-octanol. Applied Biochemistry and Biotechnology, 168, 697–707. https://doi.org/10.1007/s12010-012-9810-9
Coşkun, G., Çıplak, Z., Yıldız, N., & Mehmetoğlu, U. (2021). Immobilization of Candida antarctica lipase on nanomaterials and investigation of the enzyme activity and enantioselectivity. Applied Biochemistry and Biotechnology, 193, 430–445. https://doi.org/10.1007/s12010-020-03443-2
Zdarta, J., Meyer, A., Jesionowski, T., & Pinelo, M. (2018). A general overview of support materials for enzyme immobilization: Characteristics, properties practical utility. Catalysts, 8(2), 92. https://doi.org/10.3390/catal8020092
Yuan, S., Feng, L., Wang, K., Pang, J., Bosch, M., Lollar, C., Sun, Y., Qin, J., Yang, X., Zhang, P., Wang, Q., Zou, L., Zhang, Y., Zhang, L., Fang, Y., Li, J., & Zhou, H. C. (2018). Stable metal-organic frameworks: Design, synthesis, and applications. Advanced Materials, 30(37), 1704303. https://doi.org/10.1002/adma.201704303
Liang, K., Ricco, R., Doherty, C. M., Styles, M. J., Bell, S., Kirby, N., Mudie, S., Haylock, D., Hill, A. J., Doonan, C. J., & Falcaro, P. (2015). Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nature Communications, 6, 7240. https://doi.org/10.1038/ncomms8240
Salgaonkar, M., Nadar, S. S., & Rathod, V. K. (2019). Biomineralization of orange peel peroxidase within metal organic frameworks (OPP–MOFs) for dye degradation. Journal of Environmental Chemical Engineering, 7(2), 102969. https://doi.org/10.1016/j.jece.2019.102969
Wu, X., Yang, C., & Ge, J. (2017). Green synthesis of enzyme/metal-organic framework composites with high stability in protein denaturing solvents. Bioresources and Bioprocessing, 4, 24. https://doi.org/10.1186/s40643-017-0154-8
Du, Y., Gao, J., Zhou, L., Ma, L., He, Y., Huang, Z., & Jiang, Y. (2017). Enzyme nanocapsules armored by metal-organic frameworks: A novel approach for preparing nanobiocatalyst. Chemical Engineering Journal, 327, 1192–1197. https://doi.org/10.1016/j.cej.2017.07.021
Liang, S., Wu, X. L., Xiong, J., & Zong, M. H. (2019). Metal-organic frameworks as novel matrices for efficient enzyme immobilization: An update review. Coordination Chemistry Reviews, 406, 213149. https://doi.org/10.1016/j.ccr.2019.213149
Chen, X., Xue, S., Lin, Y., Luo, J., & Kong, L. (2020). Immobilization of porcine pancreatic lipase onto a metal-organic framework, PPL@MOF: A new platform for efficient ligand discovery from natural herbs. Analytica Chimica Acta, 1099, 94–102. https://doi.org/10.1016/j.aca.2019.11.042
Chen, J., Sun, B., Sun, C., Zhang, P., Xu, W., Liu, Y., Xiong, B., & Tang, K. W. (2020). Immobilization of lipase AYS on UiO-66-NH2 metal-organic framework nanoparticles as a recyclable biocatalyst for ester hydrolysis and kinetic resolution. Separation and Purification Technology, 251, 117398. https://doi.org/10.1016/j.seppur.2020.117398
Hu, Y., Dai, L., Liu, D., & Du, W. (2018). Rationally designing hydrophobic UiO-66 support for the enhanced enzymatic performance of immobilized lipase. Green Chemistry, 20(19), 4500–4506. https://doi.org/10.1039/C8GC01284A
Nowroozi-Nejad, Z., Bahramian, B., & Hosseinkhani, S. (2019). A fast and efficient stabilization of firefly luciferase on MIL-53(Al) via surface adsorption mechanism. Research on Chemical Intermediates, 45, 2489–2501. https://doi.org/10.1007/s11164-019-03748-w
Xu, W., Jiao, L., Yan, H., Wu, Y., Chen, L., Gu, W., Du, D., Lin, Y., & Zhu, C. (2019). Glucose oxidase-integrated metal-organic framework hybrids as biomimetic cascade nanozymes for ultrasensitive glucose biosensing. ACS Applied Materials & Interfaces, 11(25), 22096–22101. https://doi.org/10.1021/acsami.9b03004
Shih, Y. H., Lo, S.-H., Yang, N. S., Singco, B., Cheng, Y. J., Wu, C. Y., Chang, I. H., Huang, H. Y., & Lin, C. H. (2012). Trypsin-immobilized metal-organic framework as a biocatalyst in proteomics analysis. ChemPlusChem, 77(11), 982–986. https://doi.org/10.1002/cplu.201200186
Shams, S., Ahmad, W., Memon, A. H., Wei, Y., Yuan, Q., & Liang, H. (2019). Facile synthesis of laccase mimic Cu/H3BTC MOF for efficient dye degradation and detection of phenolic pollutants. RSC Advances, 9(70), 40845–40854. https://doi.org/10.1039/C9RA07473B
Xia, H., Li, Z., Zhong, X., Li, B., Jiang, Y., & Jiang, Y. (2019). HKUST-1 catalyzed efficient in situ regeneration of NAD+ for dehydrogenase mediated oxidation. Chemical Engineering Science, 203, 43–53. https://doi.org/10.1016/j.ces.2019.03.076
Chen, S., Wen, L., Svec, F., Tan, T., & Lv, Y. (2017). Magnetic metal–organic frameworks as scaffolds for spatial co-location and positional assembly of multi-enzyme systems enabling enhanced cascade biocatalysis. RSC Advances, 7(34), 21205–21213. https://doi.org/10.1039/C7RA02291C
Wang, L., Zhi, W., Wan, J., Han, J., Li, C., & Wang, Y. (2019). Recyclable β-glucosidase by one-pot encapsulation with Cu-MOFs for enhanced hydrolysis of cellulose to glucose. ACS Sustainable Chemistry & Engineering, 7(3), 3339–3348. https://doi.org/10.1021/acssuschemeng.8b05489
Ding, M., & Jiang, H. L. (2020). Improving water stability of MOFs by a general surface hydrophobic polymerization. CCS Chemistry, 3(8), 1–23. https://doi.org/10.31635/ccschem.020.202000515
Yuan, B., Yin, X. Q., Liu, X. Q., Li, X. Y., & Sun, L. B. (2016). Enhanced hydrothermal stability and catalytic performance of HKUST-1 by incorporating carboxyl-functionalized attapulgite. ACS Applied Materials & Interfaces, 8(25), 16457–16464. https://doi.org/10.1021/acsami.6b04127
Nobakht, N., Faramarzi, M. A., Shafiee, A., Khoobi, M., & Rafiee, E. (2018). Polyoxometalate-metal organic framework-lipase: An efficient green catalyst for synthesis of benzyl cinnamate by enzymatic esterification of cinnamic acid. International Journal of Biological Macromolecules, 113, 8–19. https://doi.org/10.1016/j.ijbiomac.2018.02.023
Cao, Y., Wu, Z., Wang, T., Xiao, Y., Huo, Q., & Liu, Y. (2016). Immobilization of Bacillus subtilis lipase on a Cu-BTC based hierarchically porous metal-organic framework material: A biocatalyst for esterification. Dalton Transactions, 45(16), 6998–7003. https://doi.org/10.1039/C6DT00677A
Vince, R., & Hua, M. (1990). Synthesis and anti-HIV activity of carbocyclic 2’,3’-didehydro-2’,3’-dideoxy 2,6-disubstituted purine nucleosides. Journal of Medicinal Chemistry, 21(22), 17–21. https://doi.org/10.1002/chin.199022287
Gupta, R. K., Hill, A., Sawyer, A. W., Cozzi-Lepri, A., von Wyl, V., Yerly, S., Lima, V. D., Günthard, H. F., Gilks, C., & Pillay, D. (2009). Virological monitoring and resistance to first-line highly active antiretroviral therapy in adults infected with HIV-1 treated under WHO guidelines: A systematic review and meta-analysis. The Lancet Infectious Diseases, 9(7), 409–417. https://doi.org/10.1016/S1473-3099(09)70136-7
Xun, E., Wang, J., Zhang, H., Chen, G., Yue, H., Zhao, J., Wang, L., & Wang, Z. (2013). Resolution of N-hydroxymethyl vince lactam catalyzed by lipase in organic solvent. Journal of Chemical Technology and Biotechnology, 88(5), 904–909. https://doi.org/10.1002/jctb.3919
Smith, P. K., Krohn, R. I., Hermanson, G. T., Mallia, A. K., Gartner, F. H., Provenzano, M. D., Fujimoto, E. K., Goeke, N. M., Olson, B. J., & Klenk, D. C. (1985). Measurement of protein using bicinchoninic acid. Analytical Biochemistry, 150(1), 76–85. https://doi.org/10.1016/0003-2697(85)90442-7
Chen, C. S., Fujimoto, Y., Girdaukas, G., & Sih, C. J. (1982). Quantitative analyses of biochemical kinetic resolutions of enantiomers. Journal of the American Chemical Society, 104(25), 7294–7299. https://doi.org/10.1021/ja00389a064
Yang, Y., Dong, H., Wang, Y., He, C., Wang, Y., & Zhang, X. (2018). Synthesis of octahedral like Cu-BTC derivatives derived from MOF calcined under different atmosphere for application in CO oxidation. Journal of Solid State Chemistry, 258, 582–587. https://doi.org/10.1016/j.jssc.2017.11.033
Feng, Y., Jiang, H., Li, S., Wang, J., Jing, X., Wang, Y., & Chen, M. (2013). Metal–organic frameworks HKUST-1 for liquid-phase adsorption of uranium. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 431, 87–92. https://doi.org/10.1016/j.colsurfa.2013.04.032
Zhong, Z., Pang, S., Wu, Y. W., Jiang, S., & Ouyang, J. (2017). Synthesis and characterization of mesoporous Cu-MOF for laccase immobilization. Journal of Chemical Technology & Biotechnology, 92(7), 1841–1847. https://doi.org/10.1002/jctb.5189
Morellon-Sterling, R., Siar, E. H., Braham, S. A., de Andrades, D., Pedroche, J., Millán, M. D., & Fernandez-Lafuente, R. (2021). Effect of amine length in the interference of the multipoint covalent immobilization of enzymes on glyoxyl agarose beads. Journal of Biotechnology, 329, 128–142. https://doi.org/10.1016/j.jbiotec.2021.02.005
Meylan, W. M., & Howard, P. H. (2000). Estimating log P with atom/fragments and water solubility with log P. Perspectives in Drug Discovery and Design, 19, 67–84. https://doi.org/10.1023/A:1008715521862
Zaks, A., & Klibanov, A. M. (1988). The effect of water on enzyme action in organic media. Journal of Biological Chemistry, 263(17), 8017–8021. https://doi.org/10.1016/S0021-9258(18)68435-2
Wehtje, E., Costes, D., & Adlercreutz, P. (1997). Enantioselectivity of lipases: Effects of water activity. Journal of Molecular Catalysis B: Enzymatic, 3(5), 221–230. https://doi.org/10.1016/S1381-1177(97)00003-9
Phillips, R. S. (1996). Temperature modulation of the stereochemistry of enzymatic catalysis: Prospects for exploitation. Trends in Biotechnology, 14(1), 13–16. https://doi.org/10.1016/0167-7799(96)80908-5
Lin, C., Du, Y., Wang, S., Wang, L., & Song, Y. (2021). Glucose oxidase@Cu-hemin metal-organic framework for colorimetric analysis of glucose. Materials Science and Engineering: C, 118, 111511. https://doi.org/10.1016/j.msec.2020.111511
Zhu, L., Zhu, F. C., Qin, S., Wu, B., & He, B. F. (2016). Highly efficient resolution of N-hydroxymethyl vince lactam by solvent stable lipase YCJ01. Journal of Molecular Catalysis B: Enzymatic, 133, 150–156. https://doi.org/10.1016/j.molcatb.2016.12.009
Xue, T. Y., Xu, G. C., Han, R. Z., & Ni, Y. (2015). Soluble expression of (+)-γ-lactamase in Bacillus subtilis for the enantioselective preparation of abacavir precursor. Applied Biochemistry and Biotechnology, 176, 1687–1699. https://doi.org/10.1007/s12010-015-1670-7
Li, H. X., Gao, S. H., Qiu, Y., Liang, C. Q., Zhu, S. Z., & Zheng, G. J. (2020). Genome mining integrating semi-rational protein engineering and nanoreactor design: Roadmap for a robust biocatalyst for industrial resolution of Vince lactam. Applied Microbiology and Biotechnology, 104, 1109–1123. https://doi.org/10.1007/s00253-019-10275-6
Zhong, X., Xia, H., Huang, W., Li, Z., & Jiang, Y. (2020). Biomimetic metal-organic frameworks mediated hybrid multi-enzyme mimic for tandem catalysis. Chemical Engineering Journal, 381, 122758. https://doi.org/10.1016/j.cej.2019.122758
Funding
The authors are grateful for the financial support from the Young and Middle-aged Scientific and Technological Innovation Leading Talents and Teams of Jilin Province (no. 20200301029RQ), Jilin COFCO Biochemical Co., Ltd. (2018220002000466), and the Fund of Scientific Research from the Education Department of Jilin Province (JJKH20210170KJ).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Qiaojuan Cheng: material preparation, data collection, data analysis and writing original draft. Xinyu Chi, Yingchao Liang, Wanxin Li, Jiaxin Sun, and Jin Tao: supervision of the work. Zhi Wang: reviewing and editing of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, Q., Chi, X., Liang, Y. et al. Immobilization of Lipase in Cu-BTC MOF with Enhanced Catalytic Performance for Resolution of N-hydroxymethyl Vince Lactam. Appl Biochem Biotechnol 195, 1216–1230 (2023). https://doi.org/10.1007/s12010-022-04212-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04212-z