Abstract
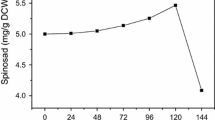
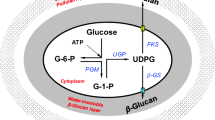
Phosphate concentration above 10 mM reduces the production of many secondary metabolites; however, the phenomenon is not mechanistically understood yet. Specifically, the problem of phosphorus limitation in antibiotic production remains unresolved. This study investigates the phosphorus inhibition effect on spinosad production and alleviates it by calcium and phosphate supplementation to fermentation media. Furthermore, we examined the mechanism of fatty acids–induced increase in polyketides production. Four phosphates that were supplemented into the fermentation media include NaH2PO4, Na2HPO4, KH2PO4, and K2HPO4 and NaH2PO4 was found to be the most effective phosphate. Under the optimal phosphate condition of supplementing 20 mM NaH2PO4 on the fourth day and 5 g/L CaCO3, the maximal spinosad production reached 520 mg/L, showing a 1.65-fold increase over the control treatment. In the NaH2PO4-CaCO3 system, the de novo fatty acid biosynthesis was significantly downregulated while spinosad biosynthesis and β-oxidation were upregulated. The coordination of de novo fatty acid biosynthesis and β-oxidation promoted intracellular acetyl-CoA concentration. The results demonstrate that NaH2PO4-CaCO3 combined addition is a simple and effective strategy to alleviate phosphorus inhibition effect through the regulation of fatty acid metabolism and accumulation of immediate precursors. This information improves our understanding of phosphates’ influence on the large-scale production of polyketides.






Similar content being viewed by others
Data Availability
All datasets generated for this study are included in the article.
References
Boeck, L. D., Chio, H., Eaton, T. E., Godfrey, O. W., Jr., Michel, K. H., Nakatsukasa, W. M., & Yao, R. C. (1996). Insecticide and miticide A83543 compounds and their method of production by fermentation. Official Gazette of the United States Patent and Trademark Office Patents, 1184(1), 440.
Boynton, Z. L., Bennett, G. N., & Rudolph, F. B. (1994). Intracellular concentrations of coenzyme A and its derivatives from clostridium acetobutylicum ATCC 824 and their roles in enzyme regulation. Applied and Environmental Microbiology, 60, 39–44.
Cleveland, C. B., Bormett, G. A., Saunders, D. G., Powers, F. L., McGibbon, A. S., Reeves, G. L., Rutherford, L., & Balcer, J. L. (2002). Environmental fate of spinosad. 1. Dissipation and degradation in aqueous systems. Journal of Agricultural and Food Chemistry, 50, 3244–3256.
Dripps, J., Boucher, R., Chloridis, A., Cleveland, C., DeAmicis, C., Gomez, L., Paroonagian, D., Pavan, L., Sparks, T., & Watson, G. (2011). The spinosyn insecticides. Lopez, O., Fernandez and Bolanos, JG (Eds), Trends in insect control. Royal Society of Chemistry, Cambridge, 163–212
Fowler, Z. L., Gikandi, W. W., & Koffas, M. A. (2009). Increased malonyl coenzyme A biosynthesis by tuning the Escherichia coli metabolic network and its application to flavanone production. Applied and Environmental Microbiology, 75, 5831–5839.
Hong, L., Zhao, Z., & Liu, H.-W. (2006). Characterization of SpnQ from the spinosyn biosynthetic pathway of Saccharopolyspora spinosa: Mechanistic and evolutionary implications for C-3 deoxygenation in deoxysugar biosynthesis. Journal of the American Chemical Society, 128, 14262–14263.
Huang, K.-X., Xia, L., Zhang, Y., Ding, X., & Zahn, J. A. (2009). Recent advances in the biochemistry of spinosyns. Applied Microbiology and Biotechnology, 82, 13–23.
Huang, Y., Zhang, X., Zhao, C., Zhuang, X., Zhu, L., Guo, C., & Song, Y. (2018). Improvement of spinosad production upon utilization of oils and manipulation of β-oxidation in a high-producing Saccharopolyspora spinosa strain. Journal of Molecular Microbiology and Biotechnology, 28, 53–64.
Jiang, S., Zhu, L., & Huang, W. Y. (2005). Regulation of phosphate on the azalomycin B biosynthesis. Chinese Journal of Antibiotics, 30, 73–75.
Jin, Z., Cheng, X., & Cen, P. (2006). Effects of Glucose and Phosphate on Spinosad Fermentation by Saccharopolyspora spinosa. Chinese Journal of Chemical Engineering, 14, 542–546.
Kim, H. J., Ruszczycky, M. W., Choi, S.-H., Liu, Y.-N., & Liu, H.-W. (2011). Enzyme-catalysed [4+ 2] cycloaddition is a key step in the biosynthesis of spinosyn A. Nature, 473, 109–112.
Madduri, K., Waldron, C., Matsushima, P., Broughton, M., Crawford, K., Merlo, D., & Baltz, R. (2001). Genes for the biosynthesis of spinosyns: Applications for yield improvement in Saccharopolyspora spinosa. Journal of Industrial Microbiology and Biotechnology, 27, 399–402.
Madduri, K., Waldron, C., & Merlo, D. J. (2001). Rhamnose biosynthesis pathway supplies precursors for primary and secondary metabolism in Saccharopolyspora spinosa. Journal of Bacteriology, 183, 5632–5638.
Martín, J. F. (1977). in Advances in Biochemical Engineering, Volume 6, Springer, pp. 105–127
Martin, J. F., & Demain, A. L. (1980). Control of antibiotic biosynthesis. Microbiological Reviews, 44, 230–251.
Mertz, F. P., & Yao, R. C. (1990). Saccharopolyspora spinosa sp. nov. isolated from soil collected in a sugar mill rum still. International Journal of Systematic and Evolutionary Microbiology, 40, 34–39.
Millar, N. S., & Denholm, I. (2007). Nicotinic acetylcholine receptors: Targets for commercially important insecticides. Invertebrate Neuroscience, 7, 53–66.
Ming-Feng, P., Mei-Jin, G., Ju, C., Wei-Qun, G., & Xiao-Lin, Z. (2012). Optimization of the seed medium and fermentation medium for spinosad biosynthesis by Saccharopolyspora spinosa CB11. Chinese Journal of Antibiotics, 37, 745–751.
Ni, L. (1988). Phosphate control to rifamycin SV biosynthesis. Acta Microbiologica Sinica, 28, 340.
Pan, H.-X., Li, J.-A., He, N.-J., Chen, J.-Y., Zhou, Y.-M., Shao, L., & Chen, D.-J. (2011). Improvement of spinosad production by overexpression of gtt and gdh controlled by promoter PermE* in Saccharopolyspora spinosa SIPI-A2090. Biotechnology Letters, 33, 733–739.
Peng, C., An, D., Ding, W.-X., Zhu, Y.-X., Ye, L., & Li, J. (2020). Fungichromin production by Streptomyces sp. WP-1, an endophyte from Pinus dabeshanensis, and its antifungal activity against Fusarium oxysporum. Applied Microbiology and Biotechnology, 104, 10437–10449.
Pradhan, S., & Pokhrel, M. R. (2013). Spectrophotometric determination of phosphate in sugarcane juice, fertilizer, detergent and water samples by molybdenum blue method. Scientific World, 11, 58–62.
Rozen, S., & Skaletsky, H. (2000). Primer3 on the WWW for general users and for biologist programmers. Methods in Molecular Biology (Clifton N J), 132, 365–386.
Salgado, V. L. (1998). Studies on the mode of action of spinosad: Insect symptoms and physiological correlates. Pesticide Biochemistry and Physiology, 60, 91–102.
Santos, V. S. V., Silva, C. E., Oliveira, C. M., de Morais, C. R., Limongi, J. E., & Pereira, B. B. (2019). Evaluation of toxicity and environmental safety in use of spinosad to rationalize control strategies against Aedes aegypti. Chemosphere, 226, 166–172.
Waldron, C., Madduri, K., Crawford, K., Merlo, D. J., Treadway, P., Broughton, M. C., & Baltz, R. H. (2000). A cluster of genes for the biosynthesis of spinosyns, novel macrolide insect control agents produced by Saccharopolyspora spinosa. Antonie van Leeuwenhoek, 78, 385–390.
Xie, Y., & Wang, G. (2015). Mechanisms of fatty acid synthesis in marine fungus-like protists. Applied Microbiology and Biotechnology, 99, 8363–8375.
Xue, C., Zhang, X., Yu, Z., Zhao, F., Wang, M., & Lu, W. (2013). Up-regulated spinosad pathway coupling with the increased concentration of acetyl-CoA and malonyl-CoA contributed to the increase of spinosad in the presence of exogenous fatty acid. Biochemical Engineering Journal, 81, 47–53.
Zhang, Y. H., Liu, H. X., Hong, C., Feng, Z., Tang, J. L., & Xia, L. Q. (2016). Effect of inorganic salts on the fermentation yield of spinosad by Saccharopolyspora spinosa. Chinese Journal of Antibiotics, 41, 658–665.
Funding
This research was financially supported by the National Natural Science Foundation of China (Nos. 21937002,), and the National Science and Technology Major Project for New Drug Research and Development of MOST (2019ZX09735002-005).
Author information
Authors and Affiliations
Contributions
CP performed the data analysis and wrote the first draft of the manuscript. M-YW and CP performed the experiments and data interpretation. M-RW and W-XD provided technical assistance to CP and J-FH. J-YL supervised the experimental design, data analysis, manuscript writing, and revision. All authors read and agreed on the final text.
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wan, MY., Peng, C., Ding, WX. et al. Calcium-Phosphate Combination Enhances Spinosad Production in Saccharopolyspora spinosa via Regulation of Fatty Acid Metabolism. Appl Biochem Biotechnol 194, 2528–2541 (2022). https://doi.org/10.1007/s12010-022-03799-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03799-7