Abstract
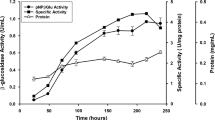
A new cellulase producer strain of Penicillium digitatum (RV 06) was previously obtained from rotten maize grains. This work aim was to optimize the production and characterize this microorganism produced cellulase. A CMCase maximum production (1.6 U/mL) was obtained in stationary liquid culture, with an initial pH of 5.0, at 25 °C, with 1% lactose as carbon source, and cultured for 5 days. The produced enzyme was purified by ammonium sulfate precipitation and exclusion chromatography. The purified enzyme optimal temperature and pH were 60 °C and 5.2, respectively. The experimental Tm of thermal inactivation was 63.68 °C, and full activity was recovered after incubation of 7 h at 50 °C. The purified 74 kDa CMCase presented KM for CMC of 11.2 mg/mL, Vmax of 0.13 μmol/min, kcat of 52 s−1, and kcat/KM of 4.7 (mg/mL)−1 s−1. The purified enzyme had a high specificity for CMC and p-nitrophenyl cellobioside and released glucose and cellobiose as final products of the CMC hydrolysis. The enzyme trypsin digestion produced peptides whose masses were obtained by MALDI-TOF/TOF mass spectrometry, which was also used to obtain two peptide sequences. These peptide sequences and the mass peak profile retrieved a CBHI within the annotated genome of P. digitatum PD1. Sequence alignments and phylogenetic analysis confirmed this enzyme as a CBHI of the glycoside hydrolase family 7. The P. digitatum PD1 protein in silico structural model revealed a coil and β-conformation predominance, which was confirmed by circular dichroism of the P. digitatum RV 06 purified enzyme.









Similar content being viewed by others
References
Singh, R., Kumar, M., Mittal, A., & Mehta, P. K. (2016). Microbial enzymes: industrial progress in 21st century. 3 Biotech, 6(2), 174.
Adrio, J. L., & Demain, A. L. (2014). Microbial enzymes: Tools for biotechnological processes. Biomolecules, 4(1), 117–139.
Kuhad, R. C., Gupta, R., & Singh, A. (2011). Microbial cellulases and their industrial applications. Enzyme Research, 2011, 280696.
Dashtban, M., Schraft, H., & Qin, W. (2009). Fungal bioconversion of lignocellulosic residues; opportunities & perspectives. International Journal of Biological Sciences, 5(6), 578–595.
Zhang, P., Himmel, M. E., & Mielenz, J. R. (2006). Outlook for cellulase improvement: Screening and selection strategies. Biotechnology Advances, 24, 452–481.
Payne, C. M., Knott, B. C., Mayes, H. B., Hansson, H., Himmel, M. E., Sandgren, M., Stahlberg, J., & Beckham, G. T. (2015). Fungal cellulases. Chemical Reviews, 115, 1308–1448.
Gusakov, A. V., & Sinitsyn, A. P. (2012). Cellulases from Penicillium species for producing fuels from biomass. Biofuels, 3(4), 463–477.
Faria, C. B., Abe, C. A. L., Silva, C. N., Tessmann, D. J., & Barbosa-Tessmann, I. P. (2012). New PCR assays for the identification of Fusarium verticillioides, Fusarium subglutinans, and other species of the Gibberella fujikuroi complex. International Journal of Molecular Sciences, 13, 115–132.
Abe, C. A. L., Faria, C. B., Castro, F. F., Souza, S. R., Santos, F. C., Silva, C. N., Tessmann, D. J., & Barbosa-Tessmann, I. P. (2015). Fungi isolated from maize (Zea mays L.) grains and production of associated enzyme activities. International Journal of Molecular Sciences, 16, 15328–15346.
Eckert, J. W., & Eaks, I. L. (1989). Postharvest disorders and diseases of citrus fruits. In W. Reuther, E. C. Calavan, & G. E. Carman (Eds.), The citrus industry (Vol. 5, pp. 179–250). Oakland: University of California Press.
Mandels, M., & Weber, J. (1969). The production of cellulases. Advances in Chemistry, 95, 391–414.
Canteri, M. G., Althaus, R. A., Virgens Filho, J. S., & Giglioti, E. A. (2001). SASM -Agri: sistema para análise e separação de médias em experimentos agrícolas pelos métodos Scoft-Knott, Tukey e Duncan. Brazilian Journal of Agrocomputation, 1, 18–24.
Ghose, T. K. (1987). Measurement of cellulase activities. Pure and Applied Chemistry, 59, 257–268.
Miller, G. L. (1959). Use of Dinitrosalicylic acid reagent for determination of deducing sugar. Analytical Chemistry, 31(3), 426–428.
Zhang, Y.-H. P., Cui, J., Lynd, L. R., & Kuang, L. R. (2006). A transition from cellulose swelling to cellulose dissolution by o-phosphoric acid: evidence from enzymatic hydrolysis and supramolecular structure. Biomacromolecules, 7(2), 644–648.
Deshpande, M. V., Eriksson, K.-E., & Pettersson, L. G. (1984). An assay for selective determination of exo-1,4,-β-glucanases in a mixture of cellulolytic enzymes. Analytical Biochemistry, 138, 48l–487l.
Bradford, M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of dye-binding. Analytical Biochemistry, 72, 248–254.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227(5259), 680–685.
Chaabouni, S. E., Mechichi, T., Limam, F., & Marzouki, N. (2005). Purification and characterization of two low molecular weight endoglucanases produced by Penicillium occitanis mutant Pol 6. Applied Biochemistry and Biotechnology, 125(2), 99–112.
Han, S. O., Yukawa, H., Inui, M., & Doi, R. H. (2005). Molecular cloning and transcriptional and expression analysis of engO, encoding a new noncellulosomal family 9 enzyme, from Clostridium cellulovorans. Journal of Bacteriology, 187(14), 4884–4889.
Trevelyan, W. E., Procter, D. P., & Harrison, J. S. (1950). Detection of sugars on paper chromatograms. Nature, 166(4219), 444–445.
Perkins, D. N., Pappin, D. J., Creasy, D. M., & Cottrell, J. S. (1999). Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis, 20(18), 3551–3567.
Kumar, S., Stecher, G., & Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33(7), 1870–1874.
Webb, B., & Sali, A. (2016). Comparative protein structure modeling using Modeller. Current Protocols in Bioinformatics, 54, 5.6.1–5.6.37.
Moroz, O. V., Maranta, M., Shaghasi, T., Harris, P. V., Wilson, K. S., & Davies, G. J. (2015). The Three-dimensional structure of the cellobiohydrolase Cel7A from Aspergillus fumigatus at 1.5 A resolution. Acta Crystallographica. Section F, Structural Biological Communications, 71(Pt 1), 114–20. L.
Collaborative Computational project, number 4. (1994). The CCP4 suite: programs for protein crystallography. Acta Crystallographica. Section D, Biological Crystallography, 50(Pt 5), 760–763.
Cheng, J., Saigo, H., & Baldi, P. (2006). Large-scale prediction of disulphide bridges using kernel methods, two-dimensional recursive neural networks, and weighted graph matching. Proteins, 62(3), 617–629.
McNicholas, S., Potterton, E., Wilson, K. S., & Noble, M. E. M. (2011). Presenting your structures: The CCP4MG molecular-graphics software. Acta Cryst, D67, 386–394.
Böhm, G., Muhr, R., & Jaenicke, R. (1992). Quantitative analysis of protein far UV circular dichroism spectra by neural networks. Protein Engineering, 5(3), 191–195.
Cantor, C. R., & Schimmel, P. R. (1980). Biophysical chemistry. San Francisco: W.H. Freeman Co..
Prasanna, H. N., Ramanjaneyulu, G., & Rajasekhar Reddy, B. R. (2016). Optimization of cellulase production by Penicillium sp. 3 Biotech, 6, 162.
Jeya, M., Joo, A.-R., Lee, K.-M., Sim, W.-I., Oh, D.-K., Kim, Y.-S., Kim, I.-W., & Lee, J.-K. (2010). Characterization of endo-β-1,4-glucanase from a novel strain of Penicillium pinophilum KMJ601. Applied Microbiology and Biotechnology, 85(4), 1005–1014.
Das, A., & Ghosh, U. (2009). Solid-state fermentation of waste cabbage of Penicillium notatum NCIM NO-923 for production and characterization of cellulases. Journal of Scientific and Industrial Research, 68, 714–718.
Sindhu, R., Suprabha, N. G., & Shashidhar, S. (2011). Media engineering for the production of cellulase from Penicillium species (SBSS 30) under solid state fermentation. Biotechnology, Bioinformatics and Bioengineering, 1(3), 343–349.
Jørgensen, H., Mørkeberg, A., Krogh, K. B. R., & Olsson, L. (2005). Production of cellulases and hemicellulases by three Penicillium species: effect of substrate and evaluation of cellulose adsorption by capillary electrophoresis. Enzyme Microbial Technology, 36, 42–48.
Pol, D., Laxman, R. S., & Rao, M. (2012). Purification and biochemical characterization of endoglucanase from Penicillium pinophilum MS 20. Indian Journal of Biochemistry & Biophysics, 49(3), 189–194.
Camassola, M., & Dillon, A. J. P. (2007). Production of cellulases and hemicellulases by Penicillium echinulatum grown on pretreated sugar cane bagasse and wheat bran in solid-state fermentation. Journal of Applied Microbiology, 3, 2196–2204.
Gao, L., Wang, F., Gao, F., Wanga, L., Zhao, J., & Qu, Y. (2011). Purification and characterization of a novel cellobiohydrolase (PdCel6A) from Penicillium decumbens JU-A10 for bioethanol production. Bioresource Technology, 102(17), 8339–8342.
Seiboth, B., Hartl, L., Pail, M., Fekete, E., Karaffa, L., & Kubicek, C. P. (2004). The galactokinase of Hypocrea jecorina is essential for cellulase induction by lactose but dispensable for growth on D-galactose. Molecular Microbiology, 51(4), 1015–1025.
Santa-Rosa, P. S., Souza, A. L., Roque, R. A., Andradea, E. V., Astolfi-Filho, S., Mota, A. J., & Nunes-Silva, C. G. (2018). Production of thermostable β-glucosidase and CMCase by Penicillium sp. LMI01 isolated from the Amazon region. Electronic Journal of Biotechnology, 31, 84–92.
Bai, H., Wang, H., Sun, J., Irfan, M., Han, M., Huang, Y., Han, X., & Yang, Q. (2013). Purification and characterization of beta 1,4-glucanases from Penicillium simplicissimum H-11. BioResources, 8(3), 3657–3671.
Das, A., Ghosh, U., Mohapatra, P. K. D., Pati, B. R., & Mondal, K. C. (2012). Study on thermodynamics and adsorption kinetics of purified endoglucanase (CMCase) from Penicillium notatum Ncim No-923 produced under mixed solid-state fermentation of waste cabbage and bagasse. Brazilian Journal of Microbiology, 43(3), 1103–1111.
Volkov, P. V., Rozhkova, A. M., Gusakov, A. V., & Sinitsyn, A. P. (2014). Homologous cloning, purification and characterization of highly active cellobiohydrolase I (Cel7A) from Penicillium canescens. Protein Expression and Purification, 103, 1–7.
Dotsenko, A. S., Gusakov, A. V., Volkov, P. V., Rozhkova, A. M., & Sinitsyn, A. P. (2016). N-linked glycosylation of recombinant cellobiohydrolase I (Cel7A) from Penicillium verruculosum and its effect on the enzyme activity. Biotechnology and Bioengineering, 113(2), 283–291.
Gao, L., Gao, F., Wang, L., Geng, C., Chi, L., Zhao, J., & Qu, Y. (2012). N-Glycoform diversity of cellobiohydrolase I from Penicillium decumbens and synergism of nonhydrolytic glycoform in cellulose degradation. The Journal of Biological Chemistry, 287(19), 15906–15915.
Divne, C., Stahlberg, J., Reinikainen, T., Ruohonen, L., Pettersson, G., Knowles, J. K., Teeri, T. T., & Jones, T. A. (1994). The three-dimensional crystal structure of the catalytic core of cellobiohydrolase I from Trichoderma reesei. Science, 265(5171), 524–528.
Jalak, J., Kurašin, M., Teugjas, H., & Väljamäe, P. (2012). Endo-exo synergism in cellulose hydrolysis. The Journal of Biological Chemistry, 287(34), 28802–28815.
Grassick, A., Murray, P. G., Thompson, R., Collins, C. M., Byrnes, L., Birrane, G., Higgins, T. M., & Tuohy, M. G. (2004). Three-dimensional structure of a thermostable native cellobiohydrolase, CBH IB, and molecular characterization of the cel7 gene from the filamentous fungus, Talaromyces emersonii. European Journal of Biochemistry, 271(22), 4495–4506.
Shoemaker, S., Schweickart, V., Ladner, M., Gelfand, D., Kwok, S., Myambo, K., & Innis, M. (1983). Molecular cloning of exo-cellobiohydrolase I derived from Trichoderma reesei strain L27. Biotechnology, 1, 691–696.
van Arsdell, J. N., Kwok, S., Schweickart, V. L., Ladner, M. B., Gelfand, D. H., & Innis, M. A. (1987). Cloning, characterization, and expression in Saccharomyces cerevisiae of endoglucanase I from Trichoderma reesei. BioTechnology, 5(1), 60–64.
Bischof, R. H., Ramoni, J., & Seiboth, B. (2016). Cellulases and beyond: the first 70 years of the enzyme producer Trichoderma reesei. Microbial Cell Factories, 15, 106.
Beckham, G. T., Dai, Z., Matthews, J. F., Momany, M., Payne, C. M., Adney, W. S., Baker, S. E., & Himmel, M. E. (2012). Harnessing glycosylation to improve cellulase activity. Current Opinion in Biotechnology, 23(3), 338–345.
Davies, G. J., Wilson, K. S., & Henrissat, B. (1997). Nomenclature for sugar-binding subsites in glycosyl hydrolases. The Biochemical Journal, 321(Pt 2), 557–559.
Henrissat, B. (1998). Glycoside families. Biochemical Society Transactions, 26(2), 153–156.
Nerinckx, W., Desmet, T., & Claeyssens, M. (2003). A hydrophobic platform as a mechanistically relevant transition state stabilizing factor appears to be present in the active centre of all glycoside hydrolases. FEBS Letters, 538(1-3), 1–7.
Colussi, F., Serpa, V., Delabona, P. S., Manzine, L. R., Voltatodio, M. L., Alves, R., Mello, B. L., Pereira Jr., N., Farinas, C. S., Golubev, A. M., Santos, M. A., & Polikarpov, I. (2011). Purification, and biochemical and biophysical characterization of cellobiohydrolase I from Trichoderma harzianum IOC 3844 J. Journal of Microbiology Biotechnology, 21(8), 808–817.
Muñoz, I. G., Ubhayasekera, W., Henriksson, H., Szabó, I., Pettersson, G., Johansson, G., Mowbray, S. L., & Ståhlberg, J. (2001). Family 7 cellobiohydrolases from Phanerochaete chrysosporium: crystal structure of the catalytic module of Cel7D (CBH58) at 1.32 Å resolution and homology models of the isozymes. Journal of Molecular Biology, 314, 1097–1111.
Acknowledgments
The authors thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Grant 001 – Ministry of Education, Brazil) for the students’ scholarships. The authors are also thankful to the Department of Biochemistry from the Federal University of Paraná, Brazil, and the Analytical Core at the State University of Maringá, for the use of its MS facility and CD equipment, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PPTX 148 kb)
Rights and permissions
About this article
Cite this article
dos Santos, F.C., de Oliveira, M.A.S., Seixas, F.A.V. et al. A Novel Cellobiohydrolase I (CBHI) from Penicillium digitatum: Production, Purification, and Characterization. Appl Biochem Biotechnol 192, 257–282 (2020). https://doi.org/10.1007/s12010-020-03307-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03307-9