Abstract
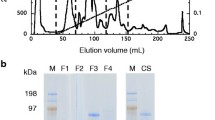
We have reported that A. pullulans 98 produces a high yield of cellulase. In this study, a carboxymethyl cellulase (CMCase) in the supernatant of the culture of A. pullulans 98 was purified to homogeneity, and the maximum production of CMCase was 4.51 U (mg protein)−1. The SDS-PAGE analysis showed that the molecular mass of the purified CMCase was 67.0 kDa. The optimal temperature of the purified enzyme with considerable thermosensitivity was 40°C, much lower than that of the CMCases from other fungi. The optimal pH of the enzyme was 5.6, and the activity profile was stable in a range of acidity (pH 5.0–6.0). The enzyme was activated by Na+, Mg2+, Ca2+, K+, Fe2+ and Cu2+, however, it was inhibited by Fe3+, Ba2+, Zn2+, Mn2+ and Ag+. K m and V max values of the purified enzyme were 4.7 mg mL−1 and 0.57 µmol L−1 min−1 (mg protein)−1, respectively. Only oligosaccharides with different sizes were released from carboxymethylcellulose (CMC) after hydrolysis with the purified CMCase. The putative gene encoding CMCase was cloned from A. pullulans 98, which contained an open reading frame of 954 bp (EU978473). The protein deduced contained the conserved domain of cellulase superfamily (glucosyl hydrolase family 5). The N-terminal amino acid sequence of the purified CMCase was M-A-P-H-A-E-P-Q-S-Q-T-T-E-Q-T-S-S-G-Q-F, which was consistent with that deduced from the cloned gene. This suggested that the purified CMCase was indeed encoded by the cloned CMCase gene in this yeast.
Similar content being viewed by others
References
Adams, A., Gottschling, D. E., Kaiser, C. A., and Stearns, T., 1998. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor Laboratory Press, New York, 84–88.
Akiba, S., Kimura, Y., Yamamoto, K., and Kumagai, H., 1995. Purification and characterization of a protease-resistant cellulase from Aspergillus niger. Journal of Fermentation and Bioengineering, 79: 25–30.
Bradford, M. M., 1976. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72: 248–253.
Chi, Z. M., Ma, C., Wang, P., and Li, H. F., 2007. Optimization of medium and cultivation conditions for alkaline protease production by the marine yeast Aureobasidium pullulans. Bioresource Technology, 98: 534–538.
Chi, Z. M., and Liu, Z. Q., 2006. Marine yeasts and their applications in mariculture. Journal of Ocean University of China, 5 (3): 243–247.
Chi, Z. M., Yan, K. R., Gao, L. M., Li, J., and Wang, X. H., 2008. Diversity of marine yeasts with high protein content and evaluation of their nutritive compositions. Journal of Marine Biology Association UK, 88: 1347–1352.
Deshpande, M. S., Rale, V. B., and Lynch, J. M., 1992. Aureobasidium pullulans in applied microbiology: A status report. Enzyme and Microbial Technology, 14: 514–527.
Enokibara, S., Mori, N., and Kitamoto, Y., 1992. Purification and some properties of a carboxymethyl cellulase from Favolus arcularius. Journal of Fermentation Bioengineering, 73: 230–232.
Federici, F., 1982. Extracellular enzymatic activities in Aureobasidium pullulans. Mycology, 74: 738–743.
Fonseca, G. G., Heinzle, E., Wittmann, C., and Gombert, A. K., 2008. The yeast Kluyveromyces marxianus and its biotechnological potential. Applied Microbiology and Biotechnology, 79: 339–354.
George, V., and Diwan, A. M., 1983. Simultaneous staining of proteins during polyacrylamide gel electrophoresis in acidic gels by countermigration of Coomassie brilliantblue R-250. Analytical Biochemistry, 132: 481–483.
Gong, F., Sheng, J., Chi, Z. M., and Li, J., 2007. Inulinase production by a marine yeast Pichia guilliermondii and inulin hydrolysis by the crude inulinase. Journal of Industrial Microbiology and Biotechnology, 34: 179–185.
Hatano, T., Komura, T., Cui, Z., Mochizuki, D., Miyakawa, T., and Matsuzaki, H., 1994. Expression of the carboxymethylcellulase gene, CMC1, from Cryptococcus flavus in Saccharomyces cerevisiae. Journal of Fermentation Bioengineering, 77: 235–238.
Ikeda, Y., Park, E. Y., and Okida, N., 2006. Bioconversion of waste office paper to gluconic acid in a turbine blade reactor by the filamentous fungus Aspergillus niger. Bioresource Technology, 97: 1030–1035.
Kim, K. C., Yoo, S. S., Oh, Y. A., and Kim, S. J., 2003. Isolation and characteristics of Trichoderma harzianum FJ1 producing cellulases and xylanase. Journal of Microbiology and Biotechnology, 13: 1–8.
Kudanga, T., and Mwenje, E., 2005. Extracellular cellulase production by tropical isolates of Aureobasidium pullulans. Microbiology, 51: 773–776.
Laemmli, U. K., 1970. Cleavage of structural proteins during assembly of head of bacteriophage T4. Nature, 227: 680–685.
Li, H. F., Chi, Z. M., Duan, X. H., Wang, L., and Sheng, J., 2007. Glucoamylase production by the marine yeast Aureobasidium pullulans N13d and hydrolysis of potato starch granules by the enzyme. Process Biochemistry, 42: 462–465.
Liu, G. L., Li, Y., Zhou, H. X., Chi, Z. M., and Madzak, C., 2012. Over-expression of a bacterial chitosanase gene in Yarrowia lipolytica and chitosan hydrolysis by the recombinant chitosanase. Journal of Molecular Catalysis B: Enzymatic, 83: 100–107.
Liu, G. L., Li, Y., Chi, Z., and Chi, Z. M., 2011a. Purification and characterization of κ-carrageenase from the marine bacterium Pseudoalteromonas porphyrae for hydrolysis of κ-carrageenan. Process Biochemistry, 46: 265–271.
Liu, G. L., Wang, D. S., Wang, L. F., Zhao, S. F., and Chi, Z. M., 2011b. Mig1 is involved in mycelia formation and expression of the genes encoding extracellular enzymes in Saccharomycopsis fibuligera A11. Fungal Genetics and Biology, 48: 904–913.
Liu, J. J., and Cao X. J., 2013. Biodegradation of micro-crystalline cellulose in pH-pH recyclable aqueous two-phase systems with water-soluble immobilized cellulase. Biochemical Engineering Journal, 79: 136–143.
Lo, C. M., Zhang, Q., Callow, N. V., and Ju, L. K., 2010. Cellulase production by continuous culture of Trichoderma reesei Rut C30 using acid hydrolysate prepared to retain more oligosaccharides for induction. Bioresource Technology, 101: 717–723.
Ma, C. L., Ni, X. M., Chi, Z. M., Ma, L. Y., and Gao, L. M., 2007. Purification and characterization of an alkaline protease from the marine yeast Aureobasidium pullulans for bioactive peptide production from different sources. Marine Biotechnology, 9: 343–351.
Ma, L. J., Li, C., Yang, Z. H., Jia, W. D., Zhang, D. Y., and Chen, S. L., 2013. Kinetic studies on batch cultivation of Trichoderma reesei and application to enhance cellulase production by fed-batch fermentation. Journal of Biotechnology, 166: 192–197.
Minamiguchi, K., Ooi, T., Kawaguchi, T., Okada, H., Murao, S., and Arai, M., 1995. Secretive expression of the Aspergillus aculeatus cellulase (FI-CMCase) by Saccharomyces cerevisiae. Journal of Fermentation Bioengineering, 79: 363–366.
Murashima, K., Nishimura, T., Nakamura, Y., Koga, J., Moriya, T., and Sumida, N., 2002. Purification and characterization of new endo-1,4-β-D-glucanases from Rhizopus oryzae. Enzyme and Microbial Technology, 30: 319–326.
Singhania, R. R., Sukumaran, R. K., Patel, A. K., Larroche, C., and Pandey, A., 2010. Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzyme Microbial Technology, 46: 541–549.
Song, C, Liu, G. L., Xu, J. L., and Chi, Z. M., 2010. Purification and characterization of extracellular β-galactosidase from the psychrotolerant yeast Guehomyces pullulans 17-1 isolated from sea sediment in Antarctica. Process Biochemistry, 45: 954–960.
Spiro, R. G., 1966. Analysis of sugars found in glycoproteins. Methods in Enzymology, 8: 3–26.
Sugumaran, K. R., Gowthami, E., Swathi, B., Elakkiya, S., Srivastava, S. N., Ravikumar, R., Gowdhaman, D., and Ponnusami, V., 2013. Production of pullulan by Aureobasidium pullulans from Asian palm kernel: A novel substrate. Carbohydrate Polymers, 92: 697–703.
Tang, B., Pan, H. B., Tang, W. J., Zhang, Q. Q., Ding, L. X., and Zhang, F. Q., 2012. Fermentation and purification of cellulose from a novel strain Rhizopus stolonifer var. reflexus TP-02, Biomass and Bioenergy, 36: 366–372.
Zhang, L., and Chi, Z. M., 2007. Screening and identification of a cellulase producing marine yeast and optimization of medium and cultivation conditions for cellulase production. Journal of Ocean University of China, 37: 101–108.
Zhou, J., Wang, Y. H., Chu, J., Zhuang, Y. P., Zhang, S. L., and Yin, P., 2008. Identification and purification of the main components of cellulases from a mutant strain of Trichoderma viride T 100-14. Bioresource Technology, 99: 6826–6833.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rong, Y., Zhang, L., Chi, Z. et al. A carboxymethyl cellulase from a marine yeast (Aureobasidium pullulans 98): Its purification, characterization, gene cloning and carboxymethyl cellulose digestion. J. Ocean Univ. China 14, 913–921 (2015). https://doi.org/10.1007/s11802-015-2574-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-015-2574-4