Abstract
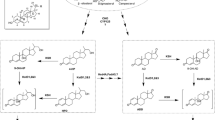
In order to obtain high 4-androstene-3,17-dione (AD) yield, nucleus degradation needs to be avoided during phytosterol bioconversion process with Mycobacterium neoaurum NwIB-R10hsd4A. 3-Ketosteroid-Δ1-dehydrogenase (KstD) catalyzes 1,2-desaturation of steroids and is a key enzyme involved in steroid nucleus oxidation. Heterogeneous expression and characterization of two KstDs (KstD2, KstD3) from M. neoaurum NwIB-R10hsd4A showed that their activities were inhibited by shifting temperature from 30 to 37 °C. However, the total activities of KstD2 and KstD3 were replenished when M. neoaurum NwIB-R10hsd4A was cultured at 37 °C because the transcription levels of kstD2 and kstD3 were upregulated 1.61- and 1.43-fold respectively compared with the cultivation at 30 °C. As the optimal temperature for cell growth was 30 °C, we developed a two-step bioprocess, cell culture at 30 °C and bioconversion with resting cells at 37 °C avoiding higher transcriptional level of kstD2 and kstD3. This process repressed the activities of KstDs, resulted in the decrease of 1,2-desaturation products, and reduced the nucleus degradation (17.6%). AD production increased to 24.7 g l−1 at higher substrate concentration (50 g l−1). These results indicated that the two-step bioprocess was potential in phytosterol biotransformation industrially.



Similar content being viewed by others
References
Fernandes, P., Cruz, A., Angelova, B., Pinheiro, H. M., & Cabral, J. M. S. (2003). Microbial conversion of steroid compounds: recent developments. Enzyme & Microbial Technology, 32(6), 688–705.
Malaviya, A., & Gomes, J. (2008). Androstenedione production by biotransformation of phytosterols. Bioresource Technology, 99(15), 6725–6737.
Donova, M. V., & Egorova, O. V. (2012). Microbial steroid transformations: current state and prospects. Applied Microbiology and Biotechnology, 94(6), 1423–1447.
Olivares, A., & Acevedo, F. (2011). Effect of inoculation strategies, substrate to biomass ratio and nitrogen sources on the bioconversion of wood sterols by Mycobacterium sp. World Journal of Microbiology and Biotechnology, 27(11), 2513–2520.
Perez, C., Falero, A., Duc, H. L., Balcinde, Y., & Hung, B. R. (2006). A very efficient bioconversion of soybean phytosterols mixtures to androstanes by Mycobacteria. Journal of Industrial Microbiology & Biotechnology, 33(8), 719–723.
Szentirmai, A. (1990). Microbial physiology of sidechain degradation of sterols. Journal of Industrial Microbiology & Biotechnology, 6(2), 101–115.
Zhang, Q. Y., Ren, Y., He, J. Z., Cheng, S. J., Yuan, J. D., Ge, F. L., Li, W., Zhang, Y., & Xie, G. (2015). Multiplicity of 3-ketosteroid Δ1-dehydrogenase enzymes in Gordonia neofelifaecis NRRL B-59395 with preferences for different steroids. Annals of Microbiology, 65(4), 1961–1971.
Bragin, E. Y., Shtratnikova, V. Y., Dovbny, D. V., Schelkunov, M. I., Pekov, Y. A., Malakho, S. G., Egorova, O. V., Ivashina, T. V., Sokolov, S. L., Ashapkin, V. V., & Donova, M. V. (2013). Comparative analysis of genes encoding key steroid core oxidation enzymes in fast-growing Mycobacterium spp. strains. Journal of Steroid Biochemistry & Molecular Biology, 138, 41–53.
Gulla, V., Banerjee, T., & Patil, S. (2010). Bioconversion of soysterols to androstenedione by Mycobacterium fortuitum subsp. fortuitum NCIM 5239, a mutant derived from total sterol degrader strain. Journal of Chemical Technology and Biotechnology, 85(8), 1135–1141.
Wei, W., Wang, F. Q., Fan, S. Y., & Wei, D. Z. (2010). Inactivation and augmentation of the primary 3-ketosteroid-Δ1-dehydrogenase in Mycobacterium neoaurum NwIB-01: biotransformation of soybean phytosterols to 4-androstene-3,17-dione or 1,4-androstadiene-3,17-dione. Applied and Environmental Microbiology, 76(13), 4578–4582.
Yao, K., Xu, L. Q., Wang, F. Q., & Wei, D. Z. (2014). Characterization and engineering of 3-ketosteroid-Δ1-dehydrogenase and 3-ketosteroid-9α-hydroxylase in Mycobacterium neoaurum ATCC 25795 to produce 9α-hydroxy-4-androstene-3, 17-dione through the catabolism of sterols. Metabolic Engineering, 24, 181–191.
Xu, L. Q., Liu, Y. J., Yao, K., Liu, H. H., Tao, X. Y., Wang, F. Q., & Wei, D. Z. (2016). Unraveling and engineering the production of 23,24-bisnorcholenic steroids in sterol metabolism. Scientific Reports, 6(1), 21928.
Xu, X. W., Gao, X. Q., Feng, J. X., Wang, X. D., & Wei, D. Z. (2015). Influence of temperature on nucleus degradation of 4-androstene-3, 17-dione in phytosterol biotransformation by Mycobacterium sp. Letters in Applied Microbiology, 61(1), 63–68.
Gao, X. Q., Feng, J. X., Wang, X. D., Hua, Q., & Wei, D. Z. (2015). Enhanced steroid metabolites production by resting cell phytosterol bioconversion. Chemical and Biochemical Engineering Quarterly, 29(4), 567–573.
Wang, Z. L., Zhao, F. S., Chen, D. J., & Li, D. T. (2006). Biotransformation of phytosterol to produce androsta-diene-dione by resting cells of Mycobacterium in cloud point system. Process Biochemistry, 41(3), 557–561.
Casabon, I., Zhu, S. H., Otani, H., Liu, J., Mohn, W. W., & Eltis, L. D. (2013). Regulation of the KstR2 regulon of Mycobacterium tuberculosis by a cholesterol catabolite. Molecular Microbiology, 89(6), 1201–1212.
Shtratnikova, V. Y., Schelkunov, M. I., Dovbnya, D. V., Eugeny, Y., Bragin, E. Y., & Donova, M. V. (2017). Effect of methyl-β-cyclodextrin on gene expression in microbial conversion of phytosterol. Applied Microbiology and Biotechnology, 101(11), 4659–4667.
Andryushina, V. A., Rodina, N. V., Stytsenko, T. S., Huy, L., Druzhinina, A. V., Yaderetz, V. V., & Voishvillo, N. E. (2011). Conversion of soybean sterols into 3,17-diketosteroids using actinobacteria Mycobacterium neoaurum, Pimelobacter simplex, and Rhodococcus erythropolis. Applied Biochemistry and Microbiology, 47(3), 270–273.
Carvalho, F., Marques, M. P. C., de Carvalho, C. C. C. R., Cabral, J. M. S., & Fernandes, P. (2009). Sitosterol bioconversion with resting cells in liquid polymer based systems. Bioresource Technology, 100(17), 4050–4053.
Zhang, X. Y., Peng, Y., Su, Z. R., Chen, Q. H., Rua, H., & He, G. Q. (2013). Optimization of biotransformation from phytosterol to androstenedione by a mutant Mycobacterium neoaurum ZJUVN-08. Journal of Zhejiang University-Science B(Biomedicine & Biotechnology), 14(2), 132–143.
Mancilla, R. A., Little, C., & Amoroso, A. (2017). Efficient bioconversion of high concentration phytosterol microdispersion to 4-Androstene-3,17-Dione (AD) by Mycobacterium sp. B3805. Applied Biochemistry and Biotechnology, 4, 1–13.
Funding
This study was funded by the National Natural Science Foundation of China (No. 21276083, 31570079).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Fig. S1
A SDS-PAGE analysis of KstDs in induced E. coli BL21(DE3). Lane 1: Marker; Lane2: KstD2, supernatant of cell lysate; Lane 3: KstD2 sediment of cell lysate; Lane 4: KstD3, supernatant of cell lysate; Lane 5: KstD3, sediment of cell lysate (PNG 182 kb)
Fig. S2
Determination of total KstDs apparent activities of Mycobacterium when cell culture was at 30 °C (■) and 37 °C (●). Consumption of AD (a) and production of ADD (b). The biotransformation were carried out in a reaction system containing 20 g l−1 cell pellets cultured at 30 °C or 37 °C, 2 g l−1 AD and 8 g l−1 HP-β-CD in 10 ml phosphate buffer (20 mmol l−1, pH 8.0) at 30 °C, with a rotary speed of 200 rpm, in a 250-ml flask. The apparent KstDs activities were determined by quantifying the amount of ADD formed in the reaction. The error bars represent mean ± SD (n = 3). (PNG 112 kb)
Fig. S3
(PNG 107 kb)
Rights and permissions
About this article
Cite this article
Wang, X., Hua, C., Xu, X. et al. Two-Step Bioprocess for Reducing Nucleus Degradation in Phytosterol Bioconversion by Mycobacterium neoaurum NwIB-R10hsd4A. Appl Biochem Biotechnol 188, 138–146 (2019). https://doi.org/10.1007/s12010-018-2895-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2895-z