Abstract
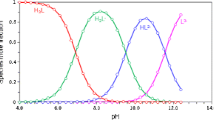
The thermodynamic dissociation constants of xylonic acid and gluconic acid were studied via potentiometric methods, and the results were verified using lactic acid, which has a known pKa value, as a model compound. Solutions of xylonic acid and gluconic acid were titrated with a standard solution of sodium hydroxide. The determined pKa data were processed via the method of derivative plots using computer software, and the accuracy was validated using the Gran method. The dissociation constants associated with the carboxylic acid group of xylonic and gluconic acids were determined to be pKa1 = 3.56 ± 0.07 and pKa1 = 3.74 ± 0.06, respectively. Further, the experimental data showed that the second deprotonation constants associated with a hydroxyl group of each of the two acids were pKa2 = 8.58 ± 0.12 and pKa2 = 7.06 ± 0.08, respectively. The deprotonation behavior of polyhydroxy carboxylic acids was altered using various ratios with Cu(II) to form complexes in solution, and this led to proposing a hypothesis for further study.








Similar content being viewed by others
References
Zhang, H., Liu, G., Zhang, J., & Bao, J. (2016). Fermentative production of high titer gluconic and xylonic acids from corn stover feedstock by Gluconobacter oxydans and techno-economic analysis. Bioresource Technology, 219, 123–131.
Zhou, X., Xu, Y., & Yu, S. (2016). Simultaneous bioconversion of xylose and glycerol to xylonic acid and 1,3-dihydroxyacetone from the mixture of pre-hydrolysates and ethanol-fermented waste liquid by Gluconobacter oxydans. Applied Biochemistry and Biotechnology, 178, 1–8.
Niu, W., Molefe, M. N., & Frost, J. W. (2003). Microbial synthesis of the energetic material precursor 1,2,4-butanetriol. Journal of the American Chemical Society, 125, 12998–12999.
Chun, B. W., Dair, B., Macuch, P. J., Wiebe, D., Porteneuve, C., & Jeknavorian, A. (2006). The development of cement and concrete additive: based on xylonic acid derived via bioconversion of xylose. Applied Biochemistry and Biotechnology, 131, 645–658.
Teglia, C. M., Brasca, R., Vera-Candioti, L., & Goicoechea, H. C. (2016). A novel approach based on capillary electrophoresis coupled to augmented multivariate curve resolution-alternating least-squares modeling for the determination of pKa of 2-hydroxy-4,6-dimethylpyrimidine in nicarbazin. Chemometrics and Intelligent Laboratory Systems, 150, 1–8.
Settimo, L., Bellman, K., & Knegtel, R. M. A. (2014). Comparison of the accuracy of experimental and predicted pKa values of basic and acidic compounds. Pharmaceutical Research, 31, 1082–1095.
Zhang, Z., Gibson, P., Clark, S. B., Tian, G., Zanonato, P. L., & Rao, L. (2007). Lactonization and protonation of gluconic acid: a thermodynamic and kinetic study by potentiometry, NMR and ESI-MS. Journal of Solution Chemistry, 36, 1187–1200.
Hummel, M., Leppikallio, M., Heikkinen, S., Niemel, K., & Sixta, H. (2010). Acidity and lactonization of xylonic acid: a nuclear magnetic resonance study. Journal of Carbohydrate Chemistry, 29, 416–428.
Yu, K., Kumar, N., Aho, A., Roine, J., Heinmaa, I., Murzin, D. Y., & Ivaska, A. (2016). Determination of acid sites in porous aluminosilicate solid catalysts for aqueous phase reactions using potentiometric titration method. Journal of Catalysis, 335, 117–124.
Xu, B., Wang, X., & Zhang, R. (2015). Improvement of data processing in determination of acetic acid ionization constant. Laboratory Science, 18, 15–18.
Motekaitis, R. J., & Martell, A. E. (1984). Complexes of aluminum (III) with hydroxy carboxylic acids. Inorganic Chemistry, 23, 18–23.
Sajadi, S. A. A. (2012). Glycine and L-tryptophan, a comparative investigation on interactions in Cu(II) binary and ternary complexes in aqueous solution. American Journal of Biochemistry, 2, 36–40.
Hagberg, J., Duker, A., & Karlsson, S. (2002). Determination of dissociation constants of low molecular weight organic acids by capillary zone electrophoresis and indirect UV detection. Chromatographia, 56, 641–644.
Greschonig, H., & Glatter, O. (1986). Determination of equivalence points of sigmoidal potentiometric titration curves. Microchimica Acta, 89, 401–409.
Kanicky, J. R., Poniatowski, A. F., Mehta, N. R., & Shah, D. O. (2000). Cooperativity among molecules at interfaces in relation to various technological processes: effect of chain length on the pKa of fatty acid salt solutions. Langmuir, 16, 172–177.
Gran, G. (1952). Determination of the equivalence point in potentiometric titrations. Part II. The Analyst, 77, 661–671.
Ivaska, A. (1973). Potentiometric titration of weak acids. Analytical Letters, 6, 961–967.
Liao, C., & Nicklaus, M. C. (2009). Comparison of nine programs predicting pK(a) values of pharmaceutical substances. Journal of Chemical Information and Modeling, 49, 2801–2812.
Meyer, A., Hoeffler, S., & Fischer, K. (2007). Anion-exchange chromatography-electrospray ionization mass spectrometry method development for the environmental analysis of aliphatic polyhydroxy carboxylic acids. Journal of Chromatography A, 1170, 62–72.
Partanen, J. I., Juusola, P. M., & Minkkinen, P. O. (2003). Determination of stoichiometric dissociation constants of lactic acid in aqueous salt solutions at 291.15 and at 298.15 K. Fluid Phase Equilibria, 204, 245–266.
Chen, Y. D., Wang, T., Helmy, R., Zhou, G. X., & LoBrutto, R. (2002). Concentration determination of methyl magnesium chloride and other Grignard reagents by potentiometric titration with in-line characterization of reaction species by FTIR spectroscopy. Journal of Pharmaceutical and Biomedical Analysis, 29, 393–404.
Silva, A. M. N., Kong, X., & Hider, R. C. (2009). Determination of the pKa value of the hydroxyl group in the α-hydroxycarboxylates citrate, malate and lactate by 13C NMR: implications for metal coordination in biological systems. Biometals, 22, 771–778.
Cho, H., Rai, D., Hess, N. J., Xia, Y. X., & Rao, L. F. (2003). Acidity and structure of isosaccharinate in aqueous solution: a nuclear magnetic resonance study. Journal of Solution Chemistry, 32, 691–702.
Matzapetakis, M., Karligiano, N., Bino, A., Dakanali, M., Raptopoulou, C. P., Tangoulis, V., Terzis, A., Giapintzakis, J., & Salifoglou, A. (2000). Manganese citrate chemistry: Syntheses, spectroscopic studies, and structural characterizations of novel mononuclear, water-soluble manganese citrate complexes. Inorganic Chemistry, 39, 4044–4051.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31370573), the Development Program of Jiangsu (BE2015758), the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institution and the Doctorate Fellowship Foundation of Nanjing Forestry University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, K., Xu, Y., Lu, W. et al. A Precise Method for Processing Data to Determine the Dissociation Constants of Polyhydroxy Carboxylic Acids via Potentiometric Titration. Appl Biochem Biotechnol 183, 1426–1438 (2017). https://doi.org/10.1007/s12010-017-2509-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2509-1