Abstract
Cell-based reporter systems have facilitated studies of viral replication and pathogenesis, virus detection, and drug susceptibility testing. There are three types of cell-based reporter systems that express certain reporter protein for positive-sense single strand RNA virus infections. The first type is classical reporter system, which relies on recombinant virus, reporter virus particle, or subgenomic replicon. During infection with the recombinant virus or reporter virus particle, the reporter protein is expressed and can be detected in real time in a dose-dependent manner. Using subgenomic replicon, which are genetically engineered viral RNA molecules that are capable of replication but incapable of producing virions, the translation and replication of the replicon could be tracked by the accumulation of reporter protein. The second type of reporter system involves genetically engineered cells bearing virus-specific protease cleavage sequences, which can sense the incoming viral protease. The third type is based on viral replicase, which can report the specific virus infection via detection of the incoming viral replicase. This review specifically focuses on the major technical breakthroughs in the design of cell-based reporter systems and the application of these systems to the further understanding and control of viruses over the past few decades.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditional diagnostic tools for positive-sense single strand RNA ((+) ssRNA) viruses, such as the virus neutralization test or virus isolation, are regarded as “gold standards” for serological and virological investigations but are also time consuming and labor intensive [11, 21]. ELISA-related assays are relatively rapid, but they are not widely used due to the low sensitivity [11]. Although nucleotide-based technologies, such as PCR and real time PCR, are more sensitive and rapid, they are expensive as well as vulnerable to false positive results arising from contaminated samples or other sources of contamination [11, 79]. Furthermore, another disadvantage of ELISA-based and nucleotide-based assays is that neither can differentiate infectious from noninfectious virus [11]. Therefore, rapid, specific live cell reporter systems for these pathogens may play important roles in monitoring infectious diseases and in virus research. The development of high throughput technologies and genetic screens have enabled widespread use of live cell-based assays for rapid sample detection, screening assays for evaluating antiviral agents and vaccines, and pathogenesis research over the past few decades. In this review, we provide an up-to-date examination of the general principles and applications of these live cell reporter systems (Fig. 1).
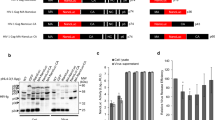
Cell-based reporter systems for (+) ssRNA virus infection. Arrow indicates activation or promotion during virus infection. ►, viral promoter; ▲, viral protease cleavage site. a Reporter system based on recombinant virus particles. Reporter gene is fused with viral structural protein or placing the reporter gene cassette behind the 3' terminus of the viral structural protein gene. Then the recombinant reporter virus is rescued and infected the target cells, and the reporter protein can be detected in the recombinant virus infected cells. b Reporter system based on virus-like particle (VLP). The reporter gene was inserted into the viral genome by replacing the structural genes to generate a replicon. The replicon RNA was transcribed in vitro and co-transfected into the cells with expression plasmids for viral structural protein, and finally the RVP was generated. Then, the target cells were infected with RVP and the reporter protein can be detected in the cells. c Reporter systems based on a viral subgenomic sequence. The reporter gene cassette controlled by viral promoter is constructed. The replicon RNA is transcribed in vitro and transfected in to the cells. Then, the reporter protein can be expressed and detected in the cells. d Protease-sensor reporter systems for (+) ssRNA virus. Sequence of viral protease cleavage site is fused with reporter gene. The expression cassette is transfected into the cells and stably expressed in the cells. During the virus infection, the cleavage site is specifically cleaved by particular virus, and the reporter protein can be detected in cells or in cells culture medium. e Replicase-sensor reporter systems for (+) ssRNA virus. The reporter gene was regulated by specific viral RdRp in the defective replicon. Without the virus infection, the replicon failed to express the reporter gene efficiently. However, when the cells were infected with the specific virus, the viral RdRp was provided by the virus and the defective replicon was activated, resulting in high-level expression of the reporter gene, which could be easily examined. R reporter protein, S structural protein, NS non-structural protein, RV recombinant virus, RVP reporter virus particle, Pro Protease, Rep Replicase, Q quenching peptide
General Principles of Live Cell Reporter Systems
Classical Reporter Systems for (+) ssRNA Virus
The first type of classical reporter systems was based on live recombinant virus carrying a reporter protein, such as enhanced green fluorescent protein (EGFP), firefly luciferase (Fluc), or secreted alkaline phosphatase (Fig. 1a and Table 1). To generate a recombinant virus, a recombinant plasmid was constructed by fusing the reporter protein with the viral structural protein or by inserting the coding sequence of reporter protein behind the 3′ terminus of the viral structural protein gene [80]. Subsequently, the recombinant virus was rescued in cells transfected with transcripts produced from the recombinant plasmid [80]. Similarly, based on an infectious complementary DNA (cDNA) clone of Dengue virus (DENV), a recombinant DENV generating luciferase was developed by engineering the luciferase gene into the capsid-coding region of an infectious cDNA clone [89]. Further studies indicated that the reporter system can be used to measure neutralization and antibody-dependent enhancement activity [71]. A classical swine fever virus (CSFV) stably expressing luciferase was developed by inserting the luciferase gene into Npro gene of CSFV [68]. The reporter virus enabled more sensitive and convenient detection of Npro protein expression and viral replication by a luciferase assay than by traditional methods [68]. The CSFV Npro was detectable as early as 4.5 h post infection [68]. Additionally, two Semliki Forest virus (SFV) constructs were developed by inserting EGFP gene or Renilla luciferase (Rluc) gene into the virus replicase open reading frame between nsP3 and nsP4 flanked by nsP2 protease-recognition sites [60, 75]. Subsequently, infectious SFV expressing detectable EGFP or Rluc upon infection were obtained by electroporation of in vitro transcripts of the constructs [60, 75].
Another strategy of classical reporter systems utilizes a reporter virus particle (RVP) (Fig. 1b). An RVP is a type of virus-like particle (VLP) composed of viral structural proteins and a self-replicating replicon RNA containing a reporter gene [17, 63]. To obtain an RVP of the West Nile virus (WNV), a red fluorescent protein gene was inserted into the WNV RNA genome by replacing the C, prM, and E protein genes to generate a WNV replicon RNA [17, 51, 55]. Subsequently, the replicon RNA was transcribed in vitro and co-transfected into cells with two DNA expression plasmids for viral C and prM/E proteins, and finally the RVP was generated [17, 55]. In a new protocol for the preparation of RVPs, the replicon RNA was stably transfected and expressed in the cells [17]. Two plasmid DNA expression plasmids for viral C and prM/E proteins were then co-transfected into the cells to package the replicon RNAs into RVPs [17]. In these RVP systems, RVPs were capable of only one round of infection in susceptible cells due to the lack of structural protein genes within the replicon RNAs [17, 87, 88]. However, RVPs are useful tools for the study of viral genome packaging and cellular factors [87].
Although recombinant viruses can be infectious, the viruses expressing fluorescent proteins tend to be attenuated due to the modification of their genomes [72]. Furthermore, many viruses are extremely virulent pathogens, such as the Middle East Respiratory Syndrome (MERS), severe acute respiratory syndrome coronavirus (SARS-CoV), and CSFV, which can only be handled in specialized laboratories of the highest biosafety level. To overcome these limitations, viral subgenomic replicons were developed and have been proven valuable for measuring the replication kinetics of replicon (Fig. 1c and Table 2). Self-replicating subgenomic replicons are genetically engineered viral RNA molecules that are shorter than full-length viral genomes and are capable of replication but incapable of producing virions [22, 29, 62, 70]. However, they can be packaged into viral particles if viral coat proteins are provided [62]. For Flaviviridae viruses, such as CSFV, two self-replicating RNAs (replicons) were produced by replacing the coding region for C to E2 of CSFV with the Rluc sequence or the coding region for C to E1 with the Rluc-2A sequence [63]. Subsequently, the translation and replication of the replicon RNAs could be followed by the accumulation of luciferase protein as well as by detection of CSFV non-structural protein (NS) 3 production within the cells [63]. Similarly, two kinds of replicons were constructed for WNV and DENV4 [3, 4]. One replicon encoded an Rluc followed by an internal ribosome entry site (IRES) element for cap-independent translation of the open reading frame encompassing the carboxy-terminal sequence of the E protein to the NS5 protein. The second replicon contained a luciferase gene, foot and mouth disease virus 2A, and a neomycin phosphotransferase gene that allows establishment of a stable mammalian cell line expressing the Rluc protein in the presence of the neomycin analog, G418 [3, 4]. The stable replicon-expressing cell line has been used for cell-based screening and determination of 50 % effective concentration (EC50) values for antiviral compounds that inhibited WNV replication [3, 4]. For hepatitis C virus (HCV), Lohmann and his colleague established efficient cell culture systems, which were based on the self replication of engineered HCV replicons [5, 6, 48]. The first one was developed by co-transfecting in vitro-transcribed HCV bicistronic replicon RNAs with the analogous mutants carrying the in-frame deletion of the NS5B polymerase active site [48]. The bicistronic replicons were composed of the HCV-IRES, the neomycin phosphotransferase (neo) gene, the IRES of the encephalomyocarditis virus, which directed translation of HCV sequences from NS2 or NS3 up to NS5B, and the 3′-untranslated regions (UTRs) [48]. However, most of these replicons focused on HCV genotype 1b, until efficient RNA replication systems for genotype 1a [7, 64] and genotype 2a [31] were established. Furthermore, to construct a tricistronic HCV replicon, two sequential IRESs were used to initiate translation of humanized Rluc and HCV non-structural genes along with an authentic HCV IRES that initiated translation of the neomycin resistance gene [12]. The results demonstrated that the tricistronic replicon was efficient to evaluate HCV infection [12]. For the Chikungunya virus (CHIKV), the replicon was constructed by replacing the structural gene of the virus with Gaussia luciferase (Gluc) gene [20]. And two helper plasmids, which contain the 5′ and 3′ CHIKV replication signals and a subgenomic promoter followed by either the capsid gene or the remaining structural proteins, were constructed expressing either the CHIKV capsid protein or envelope proteins, respectively [20]. By co-transfection of the replicon and the helper plasmids, the virus replicon particles (VRPs) could be efficiently produced [20].
Protease-Sensor Reporter Systems for (+) ssRNA Virus
Positive-sense single strand RNA virus genome encodes a large polyprotein precursor, which will be cleaved into structural and non-structural proteins by the action of cellular and viral-encoded proteases [13, 14, 32, 46]. Each virus with such a genome has a specific protease that recognizes a specific cleavage site of the polyprotein. Moreover, the cleavage of the polyprotein by the viral protease is absolutely required for assembly of the viral replicase [34], indicating that cleavage of the polyprotein depends on the particular virus infection and that the viral protease cleavage site sequence is an ideal element to report the viral infection. During the past few decades, a large number of virus-specific proteases and their cleavage sites have been identified and used to design and engineer reporter cells for specific virus infections (Fig. 1d and Table 3).
Of these viral proteases, the NS3/4A protease of HCV became an attractive target for the development of reporter systems for HCV infection. One strategy involved the fusion of EGFP to secreted alkaline phosphatase (SEAP) through the NS3/4A protease recognition sequence, Delta4AB [26, 37, 38, 41]. During HCV infection, the fused protein can be cleaved by the protease, and SEAP will be secreted into the extracellular culture medium. This strategy made it possible to monitor NS3/4A activity and replication of HCV genomic RNA inside mammalian cells by measuring SEAP levels in the culture medium [26, 37, 38, 41]. In another strategy, the recombinant caspase 3 (rCasp3) was used as the specific substrate of the NS3/4A protease; the endogenous cleavage sites in the procaspase 3 molecule were substituted with decapeptides specific for the NS3/4A protease cleavage [43]. After HCV infection, the activation of rCasp3 depended on its specific cleavage by the NS3/4A protease and resulted in apoptosis of the HCV-infected cells [43]. Additionally, Tanaka and his colleagues established an indicator cell system in which the transcriptional factors Gal4-TBP and human immunodeficiency virus type 1 (HIV-1) Tat protein were utilized [77]. The chimeric Tat and Gal4-TBP transcription factors, both containing the HCV NS3/4A cleavage sequence of a mitochondria-resident IPS-1, were manipulated to be localized in the endoplasmic reticulum. Upon infection with HCV, the transcription factors were efficiently cleaved by HCV protease, migrated into the nucleus and activated the reporter gene under the tandem promoter [77].
Another protease is the NS3 protease of CSFV. We previously developed a dark-to-bright reporter cell for CSFV infection [11]. This assay was based on a novel reporter cell stably expressing EGFP fused in-frame to a quenching peptide via a special recognition sequence of the CSFV NS3 protease [11]. Without the CSFV infection, Although EGFP can be expressed in the reporter cell, chromophore maturation of EGFP in the reporter cells was inhibited by the C-terminal quenching peptide of EGFP [56]. However, when the cells were infected with CSFV, the recognition sequence of the CSFV NS3 protease was specifically cleaved by the protease, and the quenching peptide was released from the protein. Subsequently, the EGFP can undergo conformational rearrangement allowing maturation of the chromophore and gain of fluorescence, making the cell a dark-to-bright reporter of CSFV infection [11, 56].
Similarly, a DENV-specific reporter system was created that utilized the viral protease cleavage site resulting in nuclear localization of GFP in DENV-infected cells [54]. The reporter system was based on a plasmid-containing protease cleavage site of the DENV-2 genome tagged with the SV40 NLS (nuclear localization sequence) and EGFP [54]. During DENV or RVPs infection, EGFP is transferred from the cytoplasm to the nucleus. The reporter system was shown to be effective in detecting infection of cells by all four DENV serotypes as well as by low-passaged viral strains [54]. Furthermore, these systems are also valid for the detection of VLPs, VRPs, or RVPs, and even constructs that can produce the protease RNAs.
Replicase-Sensor Reporter Systems for (+) ssRNA Virus
Positive-sense single strand RNA virus genome also encodes a RNA-dependent RNA polymerase (RdRp), which catalyzes synthesis of viral RNA and is considered as another target for virus detection and eradication [1, 2, 78]. Generally, the reporter gene was regulated by specific viral RdRp in the defective replicon (Fig. 1e). Without the virus infection, the replicon failed to express the reporter gene efficiently. However, when the cells were infected with the specific virus, the viral RdRp was provided by the virus and the defective replicon was activated, resulting in high-level expression of the reporter gene, which could be easily examined [44].
For alphaviruses, such as Sindbis virus (SINV), a stable cell line, BHKSINLuc2, containing a luciferase gene under the control of the SINV subgenomic RNA promoter, was established [58]. This cell line expressed high levels of luciferase activity during infection with SINV and provided a sensitive assay for titering the virus [58]. Moreover, a species-specific insect cell-based system for detecting wild-type SINV infection was developed, which produced SINV minigenome containing a virus inducible promoter and a fluorescent gene [72]. During the virus infection, the fluorescent gene was specially induced and expressed in the cells [72]. In another system, replicon-defective plasmids derived from the SINV were constructed by replacing the structural genes of SINV with reporter genes and deleting 1139 bp in the NS 4 gene of the virus [44]. Upon infection with the virus, the viral components induced expression of the reporter genes in the cells transfected with the replicon-defective plasmid [44].
Another replicase-sensor reporter system based on a bicistronic reporter plasmid, designated as (+)Fluc-(−)UTR-Rluc, was constructed by comprising the Fluc gene and the Rluc gene in reverse orientation flanked by both negative strands of the HCV 5′- and 3′- UTRs, in which Fluc and Rluc proteins are regulated by host polymerase and functional NS5B polymerase, respectively [39]. Then, the bicistronic plasmid was stably transfected into baby hamster kidney-21 (BHK-21) cells to generate the BHK-NS5B-FRLuc reporter cell line, which can be used to simultaneously measure cellular toxicity and intracellular RdRp activity [39]. This system is specific for the HCV RdRp activity assay and the inhibitor evaluation.
General Applications of Live Cell Reporter Systems
Reporter Systems Aimed at Virus Infections and Pathogenesis
Cell based reporter systems, combined with other genetic and proteomic approaches including RNA interference, microarray, and two-dimensional gel electrophoresis coupled with MALDI-TOF/TOF identification, have shown promise as a strategy for research on virus infections and pathogenesis. In general, the recombinant virus or RVP can mimic the virus life cycle and the reporter protein can serve as an indirect readout, thus the functions of the viral proteins and interactions of the proteins can be elucidated by measuring the reporter protein activity. Furthermore, the translation and replication of subgenomic replicon RNAs could be followed by the accumulation of luciferase protein in the cells.
Using the reporter systems, it was demonstrated that the CSFV core protein can regulate CSFV RNA synthesis by enhancing CSFV RNA-dependent RNA polymerase (NS5B) activity in a virus species-specific manner [45]. Further studies indicated that residues 21–99 of the core protein were required for enhancement of NS5B activity [45]. NS3, NS5A, and NS5B of CSFV are replication-associated proteins. However, these proteins also play an important role in internal ribosome entry site (IRES)-mediated translation of CSFV. CSFV NS3 is a multifunctional protein possessing serine protease, RNA helicase, and nucleoside triphosphatase (NTPase) activities [81]. The RNA helicase activity of NS3 could promote viral and cellular translation, whereas the protease domain of NS3 interacted with NS5B to enhance viral translation [81]. In contrast, the NS5A protein had an inhibitory effect on IRES-mediated translation, while the NS5B proteins suppressed the inhibitory effect of NS5A on viral translation by binding residues 390–414 located in the C-terminal half of NS5A [69]. Amino acids K399, T401, E406, and L413 of NS5A and aa 63–72, aa 637–653, and the highly conserved GDD motif of NS5B were necessary for the interaction between NS5A and NS5B [69, 82]. Furthermore, the 3′ terminal sequence harbored the positive and negative regulatory elements to control the IRES-mediated translation of CSFV [25]. The negative cis-acting element was the 3′-end hexamer CGGCCC sequence [25]. Moreover, mutations within IRES affected the translation initiation efficiency of CSFV [19]. Npro of CSFV was not only involved with virus RNA translation in the cytoplasm [27] but also counteracted double-stranded RNA-mediated apoptosis and IFN-α/β induction [65]. Further studies elucidated that interferon synthesis can be prevented by inhibiting transcription and promoting degradation of interferon regulatory factor 3 (IRF3) via the zinc-binding ability of viral Npro [33, 73]. Npro also redistributed to mitochondria and peroxisomes to inactivate IRF3 and inhibit apoptosis [28]. In addition, Npro influenced the innate immune response at local sites of virus replication in pigs and contributed to the pathogenicity and viral replication of CSFV [76].
Using the reporter system for DENV, it was demonstrated that the 3′ UTR, NS1, NS3, and NS5 were essential for viral RNA replication and translation. RNA replication of DENV required an RNA-RNA-mediated circularization of the viral genome, and the 5′ and 3′ UTRs formed several additional RNA elements that were involved in the regulation of translation and RNA replication [18]. The first 84 nt in the 3′ UTR comprised a variable region (VR), which could be further divided into a 5′-terminal hypervariable region (HVR) and a 3′-terminal semi-variable region (SVR) [74]. The VR was important for DENV replication and was associated with the accumulation of DENV RNA in mammalian cells [74]. The core region of the 3′-UTR of DENV RNA can form two dumbbell structures (5′- and 3′-DBs) and four pseudoknots, which were required not only for RNA replication but also for optimal translation [52]. The NS3 protein contained an N-terminal serine protease region joined to an RNA helicase by an 11-amino acid linker [50]. The linker region conferred flexibility to the NS3 protein that was required both for polyprotein processing and RNA replication [49, 50]. Furthermore, the host factor p100 can interact with the 3′ UTR to facilitate viral RNA replication [42]. Two NLSs within the central region of NS5 (‘aNLS’ and ‘bNLS’) can be recognized by the importin α/β and importin β1 nuclear transporters, respectively, leading to NS5 nuclear accumulation [61]. Moreover, the heterogeneous ribonucleoprotein (hnRNP) C1/C2 interacted with NS1 to participate in viral RNA synthesis [15, 57]. During the viral assemblying, the mature capsid protein of DENV accumulated on the surface of ER-derived lipid droplets in the cytoplasm [66]. In addition, DENV can induce low levels of interferon regulatory factor 3 and NF-κB activation by blocking the TLR-triggered ERK-NF-κB activation, thus leading to reduced cytokine production [9, 10]. Additionally, the dynamics of DENV infection in mice revealed that the virus localized predominantly to lymphoid and gut-associated tissues [67].
Reporter Systems Focused on Antiviral Research
Cell-based reporter systems also represent rapid, low cost, and high throughput assays for antiviral agent and antibody evaluation.
Replicon systems were useful for anti-viral drug development. Many anti-HCV compounds targeting NS3/4A, NS5A, or NS5B have been screened by using the HCV replicon systems, which are now available with sustained virological response up to 100 % in the majority of patients [23]. Recently, Pan and his colleagues developed a NS3/4A protease-based reporter assay, which was not only suitable for efficiently assessing HCV infection, but was also useful for high throughput screening of anti-HCV agents [59]. In this system, virus replication/infection in the cells could be quantitatively indicated by measuring the SEAP activity in the cell culture medium [59]. Similarly, a dual reporter system for WNV based on a subgenomic replicon encoding Rluc and neomycin phosphotransferase was generated [47]. Incubation of the reporting cells with a known WNV inhibitor decreased luciferase activity as well as the replicon RNA level. The efficacies of the inhibitors, as measured by the depression of luciferase activity in the reporting cells, were comparable to those derived from authentic viral infection assays, indicating that the reporting cell line can be used as a high throughput assay for anti-WNV drugs [47]. For DENV, another severe mosquito-borne viral pathogen, neither a vaccine nor an antiviral therapy is currently available to treat the disease [40]. By using cell-based reporter systems, several compounds were identified as potential antiviral agents. Two novel flavones, PMF and TMF, were discovered to have DENV-inhibitory properties [35, 36]. One effective compound of 14,400 small-molecule chemicals was found to suppress viral RNA replication [24]. A doxorubicin derivative, SA-17, can inhibit DENV replication in the very early stages of the viral replication cycle with an EC50 of 0.52 ± 0.31 μM [30]. Furthermore, nucleoside inhibitors targeting viral polymerases have proved promising for the development of drugs against viruses [40]. It has been reported that a nucleoside analog, 2′-C-methylcytidine (2CMC), exerted specific anti-DENV RNA polymerase activity in DENV subgenomic RNA replicon and infectious systems as well as in suckling mice models, with a 50 % inhibitory concentration (IC50) value of 11.2 ± 0.3 μM [40]. Moreover, BP2109 and BP13944 were identified as small-molecule inhibitors of the DENV NS2B/NS3 protease using a stable cell line harboring an efficient luciferase replicon of DENV [84–86]. Further studies elucidated that BP2109 inhibited replication and viral RNA synthesis by interacting with the central hydrophilic portion of the NS2B cofactor with an EC50 of 1.03 ± 0.09 μM, while BP13944 targeted the NS3 protease with an EC50 of 0.17 ± 0.01 μM [84, 85]. In addition, 17 new compounds were discovered as NS2B-NS3 protease inhibitors with IC50 values ranging from 7.46 ± 1.15 to 48.59 ± 3.46 μM, and 8 compounds belonging to two different scaffolds were active to some extent against DENV based on luciferase reporter replicon-based assays [16]. Additionally, amodiaquine (AQ) inhibited DENV2 infectivity with EC50 and EC90 values of 1.08 ± 0.09 μM and 2.69 ± 0.47 μM, respectively, and inhibited viral RNA replication with an EC50 value of 7.41 ± 1.09 μM in the replicon-expressing cells [8]. Both p-hydroxyanilino and diethylaminomethyl moieties were important for AQ to inhibit DENV2 replication and infectivity [8].
In the context of virus disease control, a reliable, high throughput tool for antibody or vaccine evaluation is also important to allow for an understanding of the impact of neutralizing antibodies on disease progression and vaccine efficacy [53]. A virus reporter system has emerged as a promising strategy for antibody and vaccine evaluation. A reporter system using DENV RVPs was used to measure neutralizing antibodies in human serum samples against all four DENV serotypes [53]. The results showed that DENV RVPs yielded serotype-specific responses and reproducible neutralization titers that were in statistical agreement with plaque reduction neutralization test (PRNT) results [53]. Similarly, a stable Rluc reporter system for DENV (Luc-DENV) was developed for measuring neutralization and antibody-dependent enhancement activity [71]. A luciferase value analysis using various known DENV-specific monoclonal antibodies and clinical samples from infected animals and individuals showed good repeatability and a linear correlation with conventional plaque-based assays [71]. Additionally, a rapid system to produce chimeric single-round infectious particles (SRIPs) of Flaviviruses was developed using a Japanese encephalitis virus subgenomic replicon plasmid [83]. The SRIPs of DENV-1 were evaluated as antigens for functional antibody assays [83]. As a result, a significant correlation was shown between antibody titers obtained using SRIPs and those obtained using DENV-1 antigens, indicating that SRIPs can be used as an alternative antigen in functional antibody assays [83]. A VRP-based neutralization assay for CHIKV infection was established using Gluc as readout [20]. The VRPs could be produced efficiently in the BHK-21 cells by co-transfecting of the CHIKV replicon expressing Gluc and the helper RNAs [20]. Subsequently, the infection with VRPs was measured via Gluc secreted into the supernatant, and the CHIKV-neutralizing antibodies could be determined within a day by the assay without the need of using infectious CHIKV [20].
Cell-based reporter systems are also well suited for use in clinical examinations and epidemiological surveillance [44]. The subgenomic reporter RNA systems developed by Steel and his colleagues were relatively species specific and allowed for rapid and simple visual detection of the wild-type viruses in mosquito cells [72]. Moreover, the replicon-defective reporter gene assay could detect a variety of Alphaviruses from tissue cultures with a limit of detection between one and ten (plaque-forming unit) PFU for SINV while other RNA viruses, such as the Japanese encephalitis virus and Tahyna virus, displayed negative results with this system [44].
Conclusions and Future Perspectives
Cell-based reporter systems have enhanced our understanding of the molecular mechanisms of virus replication and pathogenesis as well as virus interactions with host cells. They also provide platforms for virus detection and drug susceptibility testing (Table 4). However, there are still several issues with these systems that need to be further explored. First, not all positive-sense single strand viruses have a successful cell-based reporter system. One future challenge will be the generation of a sensitive cell-based reporter system for detecting or tracking emerging viruses and pathogenic viruses that are currently not cultivable. Second, viruses are highly variable and undergo frequent mutation, so new reporter systems for these viruses need to be developed that can identify mutations and serotypes more efficiently and accurately. Additionally, future challenges will lie in optimizing cell-based reporter systems for high throughput screening of antiviral agents.
References
Ahlquist, P. (2002). RNA-dependent RNA polymerases, viruses, and RNA silencing. Science, 296, 1270–1273.
Ahmed-Belkacem, A., Guichou, J. F., Brillet, R., Ahnou, N., Hernandez, E., Pallier, C., & Pawlotsky, J. M. (2014). Inhibition of RNA binding to hepatitis C virus RNA-dependent RNA polymerase: a new mechanism for antiviral intervention. Nucleic Acids Research, 42, 9399–9409.
Alcaraz-Estrada, S. L., Del Angel, R., & Padmanabhan, R. (2014). Construction of self-replicating subgenomic dengue virus 4 (DENV4) replicon. Methods in Molecular Biology, 1138, 131–150.
Alcaraz-Estrada, S. L., Reichert, E. D., & Padmanabhan, R. (2013). Construction of self-replicating subgenomic West Nile virus replicons for screening antiviral compounds. Methods in Molecular Biology, 1030, 283–299.
Bartenschlager, R. (2005). The hepatitis C virus replicon system: from basic research to clinical application. Journal of Hepatology, 43, 210–216.
Bartenschlager, R., & Lohmann, V. (2001). Novel cell culture systems for the hepatitis C virus. Antiviral Research, 52, 1–17.
Blight, K. J., McKeating, J. A., Marcotrigiano, J., & Rice, C. M. (2003). Efficient replication of hepatitis C virus genotype 1a RNAs in cell culture. Journal of Virology, 77, 3181–3190.
Boonyasuppayakorn, S., Reichert, E. D., Manzano, M., Nagarajan, K., & Padmanabhan, R. (2014). Amodiaquine, an antimalarial drug, inhibits dengue virus type 2 replication and infectivity. Antiviral Research, 106, 125–134.
Chang, T. H., Chen, S. R., Yu, C. Y., Lin, Y. S., Chen, Y. S., Kubota, T., Matsuoka, M., & Lin, Y. L. (2012). Dengue virus serotype 2 blocks extracellular signal-regulated kinase and nuclear factor-kappaB activation to downregulate cytokine production. PLoS One, 7, e41635.
Chang, T. H., Liao, C. L., & Lin, Y. L. (2006). Flavivirus induces interferon-beta gene expression through a pathway involving RIG-I-dependent IRF-3 and PI3K-dependent NF-kappaB activation. Microbes and Infection, 8, 157–171.
Chen, F., Yang, X., Pang, D., Peng, Z., Ma, T., Ouyang, H., & Ren, L. (2015). A dark-to-bright reporter cell for classical swine fever virus infection. Antiviral Research, 117, 44–51.
Cheng, X., Gao, X. C., Wang, J. P., Yang, X. Y., Wang, Y., Li, B. S., Kang, F. B., Li, H. J., Nan, Y. M., & Sun, D. X. (2014). Tricistronic hepatitis C virus subgenomic replicon expressing double transgenes. World Journal of Gastroenterology, 20, 18284–18295.
Curry, S., Roque-Rosell, N., Sweeney, T. R., Zunszain, P. A., & Leatherbarrow, R. J. (2007). Structural analysis of foot-and-mouth disease virus 3C protease: a viable target for antiviral drugs? Biochemical Society Transactions, 35, 594–598.
Curry, S., Roque-Rosell, N., Zunszain, P. A., & Leatherbarrow, R. J. (2007). Foot-and-mouth disease virus 3C protease: recent structural and functional insights into an antiviral target. International Journal of Biochemistry and Cell Biology, 39, 1–6.
Dechtawewat, T., Songprakhon, P., Limjindaporn, T., Puttikhunt, C., Kasinrerk, W., Saitornuang, S., Yenchitsomanus, P. T., & Noisakran, S. (2015). Role of human heterogeneous nuclear ribonucleoprotein C1/C2 in dengue virus replication. Virology Journal, 12, 14.
Deng, J., Li, N., Liu, H., Zuo, Z., Liew, O. W., Xu, W., Chen, G., Tong, X., Tang, W., Zhu, J., Zuo, J., Jiang, H., Yang, C. G., Li, J., & Zhu, W. (2012). Discovery of novel small molecule inhibitors of dengue viral NS2B-NS3 protease using virtual screening and scaffold hopping. Journal of Medicinal Chemistry, 55, 6278–6293.
Fernandez, I. V., Okamoto, N., Ito, A., Fukuda, M., Someya, A., Nishino, Y., Sasaki, N., & Maeda, A. (2014). Development of a novel protocol for generating flavivirus reporter particles. Journal of Virological Methods, 208, 96–101.
Friebe, P., Pena, J., Pohl, M. O., & Harris, E. (2012). Composition of the sequence downstream of the dengue virus 5′ cyclization sequence (dCS) affects viral RNA replication. Virology, 422, 346–356.
Friis, M. B., Rasmussen, T. B., & Belsham, G. J. (2012). Modulation of translation initiation efficiency in classical swine fever virus. Journal of Virology, 86, 8681–8692.
Glasker, S., Lulla, A., Lulla, V., Couderc, T., Drexler, J. F., Liljestrom, P., Lecuit, M., Drosten, C., Merits, A., & Kummerer, B. M. (2013). Virus replicon particle based Chikungunya virus neutralization assay using Gaussia luciferase as readout. Virology Journal, 10, 235.
Greiser-Wilke, I., Blome, S., & Moennig, V. (2007). Diagnostic methods for detection of Classical swine fever virus--status quo and new developments. Vaccine, 25, 5524–5530.
Hoenen, T., Groseth, A., de Kok-Mercado, F., Kuhn, J. H., & Wahl-Jensen, V. (2011). Minigenomes, transcription and replication competent virus-like particles and beyond: reverse genetics systems for filoviruses and other negative stranded hemorrhagic fever viruses. Antiviral Research, 91, 195–208.
Horscroft, N., Lai, V. C., Cheney, W., Yao, N., Wu, J. Z., Hong, Z., & Zhong, W. (2005). Replicon cell culture system as a valuable tool in antiviral drug discovery against hepatitis C virus. Antiviral Chemistry and Chemotherapy, 16, 1–12.
Hsu, Y. C., Chen, N. C., Chen, P. C., Wang, C. C., Cheng, W. C., & Wu, H. N. (2012). Identification of a small-molecule inhibitor of dengue virus using a replicon system. Archives of Virology, 157, 681–688.
Huang, S. W., Chan, M. Y., Hsu, W. L., Huang, C. C., & Tsai, C. H. (2012). The 3′-terminal hexamer sequence of classical swine fever virus RNA plays a role in negatively regulating the IRES-mediated translation. PLoS One, 7, e33764.
Iro, M., Witteveldt, J., Angus, A. G., Woerz, I., Kaul, A., Bartenschlager, R., & Patel, A. H. (2009). A reporter cell line for rapid and sensitive evaluation of hepatitis C virus infectivity and replication. Antiviral Research, 83, 148–155.
Jefferson, M., Donaszi-Ivanov, A., Pollen, S., Dalmay, T., Saalbach, G., & Powell, P. P. (2014). Host factors that interact with the pestivirus N-terminal protease, Npro, are components of the ribonucleoprotein complex. Journal of Virology, 88, 10340–10353.
Jefferson, M., Whelband, M., Mohorianu, I., & Powell, P. P. (2014). The pestivirus N terminal protease N(pro) redistributes to mitochondria and peroxisomes suggesting new sites for regulation of IRF3 by N(pro.). PLoS One, 9, e88838.
Jones, C. T., Patkar, C. G., & Kuhn, R. J. (2005). Construction and applications of yellow fever virus replicons. Virology, 331, 247–259.
Kaptein, S. J., De Burghgraeve, T., Froeyen, M., Pastorino, B., Alen, M. M., Mondotte, J. A., Herdewijn, P., Jacobs, M., de Lamballerie, X., Schols, D., Gamarnik, A. V., Sztaricskai, F., & Neyts, J. (2010). A derivate of the antibiotic doxorubicin is a selective inhibitor of dengue and yellow fever virus replication in vitro. Antimicrobial Agents and Chemotherapy, 54, 5269–5280.
Kato, T., Date, T., Miyamoto, M., Furusaka, A., Tokushige, K., Mizokami, M., & Wakita, T. (2003). Efficient replication of the genotype 2a hepatitis C virus subgenomic replicon. Gastroenterology, 125, 1808–1817.
Kwong, A. D., Kim, J. L., Rao, G., Lipovsek, D., & Raybuck, S. A. (1999). Hepatitis C virus NS3/4A protease. Antiviral Research, 41, 67–84.
La Rocca, S. A., Herbert, R. J., Crooke, H., Drew, T. W., Wileman, T. E., & Powell, P. P. (2005). Loss of interferon regulatory factor 3 in cells infected with classical swine fever virus involves the N-terminal protease, Npro. Journal of Virology, 79, 7239–7247.
Lai, H., Teramoto, T., & Padmanabhan, R. (2014). Construction of dengue virus protease expression plasmid and in vitro protease assay for screening antiviral inhibitors. Methods in Molecular Biology, 1138, 345–360.
Leardkamolkarn, V., & Sirigulpanit, W. (2012). Establishment of a stable cell line coexpressing dengue virus-2 and green fluorescent protein for screening of antiviral compounds. Journal of Biomolecular Screening, 17, 283–292.
Leardkamolkarn, V., Sirigulpanit, W., Chotiwan, N., Kumkate, S., & Huang, C. Y. (2012). Development of Dengue type-2 virus replicons expressing GFP reporter gene in study of viral RNA replication. Virus Research, 163, 552–562.
Lee, J. C., Chang, C. F., Chi, Y. H., Hwang, D. R., & Hsu, J. T. (2004). A reporter-based assay for identifying hepatitis C virus inhibitors based on subgenomic replicon cells. Journal of Virological Methods, 116, 27–33.
Lee, J. C., Shih, Y. F., Hsu, S. P., Chang, T. Y., Chen, L. H., & Hsu, J. T. (2003). Development of a cell-based assay for monitoring specific hepatitis C virus NS3/4A protease activity in mammalian cells. Analytical Biochemistry, 316, 162–170.
Lee, J. C., Tseng, C. K., Chen, K. J., Huang, K. J., Lin, C. K., & Lin, Y. T. (2010). A cell-based reporter assay for inhibitor screening of hepatitis C virus RNA-dependent RNA polymerase. Analytical Biochemistry, 403, 52–62.
Lee, J. C., Tseng, C. K., Wu, Y. H., Kaushik-Basu, N., Lin, C. K., Chen, W. C., & Wu, H. N. (2015). Characterization of the activity of 2′-C-methylcytidine against dengue virus replication. Antiviral Research, 116, 1–9.
Lee, J. C., Yu, M. C., Lien, T. W., Chang, C. F., & Hsu, J. T. (2005). High-throughput cell-based screening for hepatitis C virus NS3/4A protease inhibitors. Assay and Drug Development Technologies, 3, 385–392.
Lei, Y., Huang, Y., Zhang, H., Yu, L., Zhang, M., & Dayton, A. (2011). Functional interaction between cellular p100 and the dengue virus 3′ UTR. Journal of General Virology, 92, 796–806.
Lei, Y. F., Yin, W., Yang, J., Lv, X., Wei, S. H., An, Q. X., Hu, X. B., & Xu, Z. K. (2008). Development of a cell-based assay for monitoring hepatitis C virus ns3/4a protease activity. Acta Virologica, 52, 133–141.
Li, J., Zhu, W., Wang, H., Li, J., Zhang, Q., He, Y., Li, J., Fu, J., Li, D., & Liang, G. (2012). Rapid, specific detection of alphaviruses from tissue cultures using a replicon-defective reporter gene assay. PLoS One, 7, e33007.
Li, W., Zhang, Y., & Kao, C. C. (2014). The classic swine fever virus (CSFV) core protein can enhance de novo-initiated RNA synthesis by the CSFV polymerase NS5B. Virus Genes, 49, 106–115.
Li, X. D., Sun, L., Seth, R. B., Pineda, G., & Chen, Z. J. (2005). Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proceedings of the National Academy of Sciences of the United States of America, 102, 17717–17722.
Lo, M. K., Tilgner, M., & Shi, P. Y. (2003). Potential high-throughput assay for screening inhibitors of West Nile virus replication. Journal of Virology, 77, 12901–12906.
Lohmann, V., Korner, F., Koch, J., Herian, U., Theilmann, L., & Bartenschlager, R. (1999). Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science, 285, 110–113.
Luo, D., Wei, N., Doan, D. N., Paradkar, P. N., Chong, Y., Davidson, A. D., Kotaka, M., Lescar, J., & Vasudevan, S. G. (2010). Flexibility between the protease and helicase domains of the dengue virus NS3 protein conferred by the linker region and its functional implications. Journal of Biological Chemistry, 285, 18817–18827.
Luo, D., Xu, T., Hunke, C., Gruber, G., Vasudevan, S. G., & Lescar, J. (2008). Crystal structure of the NS3 protease-helicase from dengue virus. Journal of Virology, 82, 173–183.
Maeda, J., Takagi, H., Hashimoto, S., Kurane, I., & Maeda, A. (2008). A PCR-based protocol for generating West Nile virus replicons. Journal of Virological Methods, 148, 244–252.
Manzano, M., Reichert, E. D., Polo, S., Falgout, B., Kasprzak, W., Shapiro, B. A., & Padmanabhan, R. (2011). Identification of cis-acting elements in the 3′-untranslated region of the dengue virus type 2 RNA that modulate translation and replication. Journal of Biological Chemistry, 286, 22521–22534.
Mattia, K., Puffer, B. A., Williams, K. L., Gonzalez, R., Murray, M., Sluzas, E., Pagano, D., Ajith, S., Bower, M., Berdougo, E., Harris, E., & Doranz, B. J. (2011). Dengue reporter virus particles for measuring neutralizing antibodies against each of the four dengue serotypes. PLoS One, 6, e27252.
Medin, C. L., Valois, S., Patkar, C. G., & Rothman, A. L. (2015). A plasmid-based reporter system for live cell imaging of dengue virus infected cells. Journal of Virological Methods, 211, 55–62.
Moritoh, K., Maeda, A., Nishino, T., Sasaki, N., & Agui, T. (2011). Development and application of West Nile virus subgenomic replicon RNA expressing secreted alkaline phosphatase. Journal of Veterinary Medical Science, 73, 683–686.
Nicholls, S. B., & Hardy, J. A. (2013). Structural basis of fluorescence quenching in caspase activatable-GFP. Protein Sciences, 22, 247–257.
Noisakran, S., Sengsai, S., Thongboonkerd, V., Kanlaya, R., Sinchaikul, S., Chen, S. T., Puttikhunt, C., Kasinrerk, W., Limjindaporn, T., Wongwiwat, W., Malasit, P., & Yenchitsomanus, P. T. (2008). Identification of human hnRNP C1/C2 as a dengue virus NS1-interacting protein. Biochemical and Biophysical Research Communications, 372, 67–72.
Olivo, P. D., Frolov, I., & Schlesinger, S. (1994). A cell line that expresses a reporter gene in response to infection by Sindbis virus: a prototype for detection of positive strand RNA viruses. Virology, 198, 381–384.
Pan, K. L., Lee, J. C., Sung, H. W., Chang, T. Y., & Hsu, J. T. (2009). Development of NS3/4A protease-based reporter assay suitable for efficiently assessing hepatitis C virus infection. Antimicrobial Agents and Chemotherapy, 53, 4825–4834.
Pohjala, L., Barai, V., Azhayev, A., Lapinjoki, S., & Ahola, T. (2008). A luciferase-based screening method for inhibitors of alphavirus replication applied to nucleoside analogues. Antiviral Research, 78, 215–222.
Pryor, M. J., Rawlinson, S. M., Butcher, R. E., Barton, C. L., Waterhouse, T. A., Vasudevan, S. G., Bardin, P. G., Wright, P. J., Jans, D. A., & Davidson, A. D. (2007). Nuclear localization of dengue virus nonstructural protein 5 through its importin alpha/beta-recognized nuclear localization sequences is integral to viral infection. Traffic, 8, 795–807.
Queiroz, S. R., Silva, A. N., Santos, J. J., Marques, E. T., Jr., Bertani, G. R., & Gil, L. H. (2013). Construction of yellow fever virus subgenomic replicons by yeast-based homologous recombination cloning technique. Anais da Academia Brasileira de Ciências, 85, 159–168.
Risager, P. C., Fahnoe, U., Gullberg, M., Rasmussen, T. B., & Belsham, G. J. (2013). Analysis of classical swine fever virus RNA replication determinants using replicons. Journal of General Virology, 94, 1739–1748.
Robinson, M., Yang, H., Sun, S. C., Peng, B., Tian, Y., Pagratis, N., Greenstein, A. E., & Delaney, W. E. (2010). Novel hepatitis C virus reporter replicon cell lines enable efficient antiviral screening against genotype 1a. Antimicrobial Agents and Chemotherapy, 54, 3099–3106.
Ruggli, N., Bird, B. H., Liu, L., Bauhofer, O., Tratschin, J. D., & Hofmann, M. A. (2005). N(pro) of classical swine fever virus is an antagonist of double-stranded RNA-mediated apoptosis and IFN-alpha/beta induction. Virology, 340, 265–276.
Samsa, M. M., Mondotte, J. A., Iglesias, N. G., Assuncao-Miranda, I., Barbosa-Lima, G., Da Poian, A. T., Bozza, P. T., & Gamarnik, A. V. (2009). Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathogens, 5, e1000632.
Schoggins, J. W., Dorner, M., Feulner, M., Imanaka, N., Murphy, M. Y., Ploss, A., & Rice, C. M. (2012). Dengue reporter viruses reveal viral dynamics in interferon receptor-deficient mice and sensitivity to interferon effectors in vitro. Proceedings of the National Academy of Sciences of the United States of America, 109, 14610–14615.
Shen, L., Li, Y., Chen, J., Li, C., Huang, J., Luo, Y., Sun, Y., Li, S., & Qiu, H. J. (2014). Generation of a recombinant classical swine fever virus stably expressing the firefly luciferase gene for quantitative antiviral assay. Antiviral Research, 109, 15–21.
Sheng, C., Wang, J., Xiao, J., Xiao, J., Chen, Y., Jia, L., Zhi, Y., Li, G., & Xiao, M. (2012). Classical swine fever virus NS5B protein suppresses the inhibitory effect of NS5A on viral translation by binding to NS5A. Journal of General Virology, 93, 939–950.
Shi, P. Y., Tilgner, M., & Lo, M. K. (2002). Construction and characterization of subgenomic replicons of New York strain of West Nile virus. Virology, 296, 219–233.
Song, K. Y., Zhao, H., Jiang, Z. Y., Li, X. F., Deng, Y. Q., Jiang, T., Zhu, S. Y., Shi, P. Y., Zhang, B., Zhang, F. C., Qin, E. D., & Qin, C. F. (2014). A novel reporter system for neutralizing and enhancing antibody assay against dengue virus. BMC Microbiology, 14, 44.
Steel, J. J., Franz, A. W., Sanchez-Vargas, I., Olson, K. E., & Geiss, B. J. (2013). Subgenomic reporter RNA system for detection of alphavirus infection in mosquitoes. PLoS One, 8, e84930.
Szymanski, M. R., Fiebach, A. R., Tratschin, J. D., Gut, M., Ramanujam, V. M., Gottipati, K., Patel, P., Ye, M., Ruggli, N., & Choi, K. H. (2009). Zinc binding in pestivirus N(pro) is required for interferon regulatory factor 3 interaction and degradation. Journal of Molecular Biology, 391, 438–449.
Tajima, S., Nukui, Y., Takasaki, T., & Kurane, I. (2007). Characterization of the variable region in the 3′ non-translated region of dengue type 1 virus. Journal of General Virology, 88, 2214–2222.
Tamberg, N., Lulla, V., Fragkoudis, R., Lulla, A., Fazakerley, J. K., & Merits, A. (2007). Insertion of EGFP into the replicase gene of Semliki Forest virus results in a novel, genetically stable marker virus. Journal of General Virology, 88, 1225–1230.
Tamura, T., Nagashima, N., Ruggli, N., Summerfield, A., Kida, H., & Sakoda, Y. (2014). Npro of classical swine fever virus contributes to pathogenicity in pigs by preventing type I interferon induction at local replication sites. Veterinary Research, 45, 47.
Tanaka, Y., Mori, Y., Tani, H., Abe, T., Moriishi, K., Kojima, H., Nagano, T., Okabe, T., Suzuki, T., Tatsumi, M., & Matsuura, Y. (2010). Establishment of an indicator cell system for hepatitis C virus. Microbiology and Immunology, 54, 206–220.
Tseng, C. K., Chen, K. J., Lin, C. K., Hsu, S. H., & Lee, J. C. (2011). An in vitro coupled transcription/translation reporter system for hepatitis C virus RNA-dependent RNA polymerase. Analytical Biochemistry, 418, 50–57.
Wang, X., Zhang, X., Sun, Y., Li, N., He, F., Chang, T., Li, H., Yang, Z., & Qiu, H. (2010). Comparison of six detection methods for classical swine fever virus. Wei Sheng Wu Xue Bao, 50, 1087–1093.
Wuyang, Z., Li, J., Wang, H., He, Y., & Liang, G. (2013). Development of EGFP/GLUC-tagged Sindbis-like virus XJ-160. Journal of Virological Methods, 189, 235–237.
Xiao, M., Bai, Y., Xu, H., Geng, X., Chen, J., Wang, Y., Chen, J., & Li, B. (2008). Effect of NS3 and NS5B proteins on classical swine fever virus internal ribosome entry site-mediated translation and its host cellular translation. Journal of General Virology, 89, 994–999.
Xiao, M., Wang, Y., Zhu, Z., Yu, J., Wan, L., & Chen, J. (2009). Influence of NS5A protein of classical swine fever virus (CSFV) on CSFV internal ribosome entry site-dependent translation. Journal of General Virology, 90, 2923–2928.
Yamanaka, A., Suzuki, R., & Konishi, E. (2014). Evaluation of single-round infectious, chimeric dengue type 1 virus as an antigen for dengue functional antibody assays. Vaccine, 32, 4289–4295.
Yang, C. C., Hsieh, Y. C., Lee, S. J., Wu, S. H., Liao, C. L., Tsao, C. H., Chao, Y. S., Chern, J. H., Wu, C. P., & Yueh, A. (2011). Novel dengue virus-specific NS2B/NS3 protease inhibitor, BP2109, discovered by a high-throughput screening assay. Antimicrobial Agents and Chemotherapy, 55, 229–238.
Yang, C. C., Hu, H. S., Wu, R. H., Wu, S. H., Lee, S. J., Jiaang, W. T., Chern, J. H., Huang, Z. S., Wu, H. N., Chang, C. M., & Yueh, A. (2014). A novel dengue virus inhibitor, BP13944, discovered by high-throughput screening with dengue virus replicon cells selects for resistance in the viral NS2B/NS3 protease. Antimicrobial Agents and Chemotherapy, 58, 110–119.
Yang, C. C., Tsai, M. H., Hu, H. S., Pu, S. Y., Wu, R. H., Wu, S. H., Lin, H. M., Song, J. S., Chao, Y. S., & Yueh, A. (2013). Characterization of an efficient dengue virus replicon for development of assays of discovery of small molecules against dengue virus. Antiviral Research, 98, 228–241.
Yoshii, K., Goto, A., Kawakami, K., Kariwa, H., & Takashima, I. (2008). Construction and application of chimeric virus-like particles of tick-borne encephalitis virus and mosquito-borne Japanese encephalitis virus. Journal of General Virology, 89, 200–211.
Yoshii, K., Hayasaka, D., Goto, A., Kawakami, K., Kariwa, H., & Takashima, I. (2005). Packaging the replicon RNA of the Far-Eastern subtype of tick-borne encephalitis virus into single-round infectious particles: development of a heterologous gene delivery system. Vaccine, 23, 3946–3956.
Zou, G., Xu, H. Y., Qing, M., Wang, Q. Y., & Shi, P. Y. (2011). Development and characterization of a stable luciferase dengue virus for high-throughput screening. Antiviral Research, 91, 11–19.
Zhu, W., Li, J., Wang, H., He, Y., Liang, G. (2013). Development of EGFP/GLUC-tagged Sindbis-like virus XJ-160. Journal of Virological Methods 189, 235–237.
Acknowledgments
This work is supported by the Jilin Province Science and Technology Development Projects (No. 20140101123JC and 20150204077NY), the Fundamental Research Funds of Jilin University, Science and Technology Research Program during the Twelfth Five-year Plan Period of Jilin Educational Committee, and the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT1248).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Ren, L., Peng, Z., Chen, X. et al. Live Cell Reporter Systems for Positive-Sense Single Strand RNA Viruses. Appl Biochem Biotechnol 178, 1567–1585 (2016). https://doi.org/10.1007/s12010-015-1968-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1968-5