Abstract
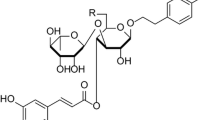
Protopine biosynthesis in Fumaria rostellata and Fumaria officinalis cell suspensions was investigated. For the first time, we reported for calli and cell suspensions obtained from F. rostellata and F. officinalis. Callus induction was initiated on a Murashige and Skoog medium, supplemented with sucrose and various concentrations of plant growth regulators: 2,4-dichlorophenoxyacetic acid (2,4-D) and 6-benzylaminopurine (BAP). The best morphological characteristics, growth behavior, and protopine biosynthesis were observed for two callus lines (5FRL14 and 12FOL1) cultivated under submerged conditions, at low concentration of 2,4-D (0.2 and 0.5 mg/L) and higher concentration of BAP (2.0 and 3.0 mg/L). The maximal yield of protopine was accumulated from cell suspension of F. rostellata (line 5FRL14) cultivated under illumination—49.6 mg/L. Time courses of utilization of sucrose, ammonium, nitrate, and phosphate ions in cultural liquid and acetylcholinesterase inhibitory activity of alkaloid extracts of studied suspensions are also presented.






Similar content being viewed by others
References
Saeed, S. A., Gilani, A. H., Majoo, R. U., & Shah, B. H. (1997). Anti-thrombotic and anti-inflammatory activities of protopine. Pharmacological Research, 36(1), 1–7.
Shen, G. Y., Chen, W. R., Midtgaard, J., Shepherd, G. M., & Hines, M. L. (1999). Computational analysis of action potential initiation in mitral cell soma and dendrites based on dual patch recordings. Journal of Neurophysiology, 82(6), 3006–3020.
Li, B., Wu, Q., Shi, J. S., Sun, A. S., & Huang, X. N. (2005). Effects of protopine on intracellular calcium and the PKC activity of rat aorta smooth muscle. Acta Pharmacologica Sinica, 57, 240–246.
Cosar, G., Bilgehan, H., & Gozler, T. (1981). The antibacterial effects of some alkaloids isolated from Glaucium fravum Crantz. Mikrobiyoloji Bülteni, 15, 105–109.
Ustunes, L., Laekeman, G. M., Gozler, B., Vlietinck, A. J., Ozer, A., & Herman, A. G. (1988). In vitro study of the anticholinergic and antihistaminic activities of protopine and some derivates. Journal of Natural Products, 51, 1021–1022.
Lee, K. H., Huh, J. W., Choi, M. M., Yoon, S. Y., Yang, S. J., Hong, H. N., & Cho, S. W. (2005). Regulation of glutamate level in rat brain through activation of glutamate dehydrogenase by Corydalis ternate. Experimental and Molecular Medicine, 37(4), 371–377.
Hu, X. Y., Sun, A. S., Yu, L. M., & Wu, Q. (2005). Effects of Corydalis ambailis migo total alkaloids on experimental cerebral ischemia. Zhong Xi Yi Jie He Xue Bao, 3, 46–49.
Suau, R., Cabezudo, B., Rico, R., Nájera, F., & López-Romero, J. M. (2002). Direct determination of alkaloid contents in Fumaria species by GC-MS. Phytochemical Analysis, 13, 363–367.
Suau, R., Cabezudo, B., Rico, R., López-Romero, J. M., & Náera, F. (2002). Alkaloids from Fumaria sepium and Fumaria agrarian. Biochemical Systematics and Ecology, 30, 263–265.
Assyov, B., Petrova, A., Dimitrov, D., & Vassilev, R. (2012). Conspectus of the Bulgarian Vascular flora. Distribution maps and floristic elements (4th ed.). Sofia: Bulgarian Biodiversity Foundation.
Tanahashi, T., & Zenk, M. H. (1985). Isoquinoline alkaloids from cell suspension cultures of Fumaria capreolata. Plant Cell Reports, 4(2), 96–99.
Sousek, J., Guèdon, D., Adam, T., Bochoràkova, H., Tàborskà, E., Vàlka, I., & Simànek, V. (1999). Alkaloids and organic acids content of eight Fumaria Species. Phytochemical Analysis, 10(1), 6–11.
Maiza-Benabdesselam, F., Chibane, M., Madani, K., Max, H., & Adach, S. (2007). Determination of isoquinoline alkaloids contents in two Algerian species of Fumaria (Fumaria capreolata and Fumaria bastardy). African Journal of Biotechnology, 6(21), 2487–2492.
Ivanov, I., Vrancheva, R., Marchev, A., Petkova, N., Aneva, I., Denev, P., Georgiev, V., & Pavlov, A. (2014). Antioxidant activities and phenolic compounds in Bulgarian Fumaria species. International Journal of Current Microbiology and Applied Science, 3(2), 296–306.
Xu, L. F., Chu, W. J., Qing, X. Y., Li, S., Wang, X. S., Qing, G. W., Fei, J., & Guo, L. H. (2006). Protopine inhibits serotonin transporter and noradrenaline transporter and has the antidepressant-like effect in mice models. Neuropharmacology, 50(8), 934–940.
Orhan, I., Özçelik, B., Karaoğlu, T., & Sener, B. (2007). Antiviral and antimicrobial profiles of selected isoquinoline alkaloids from Fumaria and Corydalis species. Zeitschrift für Naturforschung. Section C, 62(1–2), 19–26.
Rathi, A., Srivastava, A. K., Shirwaikar, A., Rawat, A. K. S., & Mehrotra, S. (2008). Hepatoprotective potential of Fumaria indica Pugsley whole plant extracts, fractions and an isolated alkaloid protopine. Phytomedicine, 15(6–7), 470–477.
Vrancheva, R., Ivanov, I., Marchev, A., & Pavlov, A. (2012). Qualitative and quantitative determination of protopine in Fumaria spp. by TLC-densitometry method. Journal of Biomedicine and Biotechnology, 1(3), 255–259.
Paltinenan, R., Toiu, A., Wauters, J. N., Frederich, M., Tits, M., Angelot, L., Tamas, M., & Crisan, G. (2013). Indentification and determination of alkaloids in Fumaria Species from Romania. Digest Journal of Nanomaterials and Biostructures, 8(2), 817–824.
Ananga, A., Georgiev, V., Ochieng, J. W., Phills, B., & Tsolova, V. (2013). Production of anthocyanins in grape cell cultures: A potential source of raw material for pharmaceutical, food, and cosmetic industries. In B. Sladonja (Ed.), The Mediterranean Genetic Code - Grapevine and Olive (pp. 247–287). Intech.
Murthy, H. N., Dandin, V. S., Zhong, J. J., & Paek, K. Y. (2014). Strategies for enhanced production of plant secondary metabolites from cell and organ cultures. In N. Murthy, J. Zhong, & Y. Paek (Eds.), Production of biomass and bioactive compounds using bioreactor technology (pp. 471–508). Netherlands: Springer.
Dixon, R. A. (1985). Isolation and maintenance of callus and cell suspension cultures in plant cell culture. In R. A. Dixon (Ed.), A practical approach (pp. 1–20). Oxford.
Pavlov, A., Georgiev, V., & Ilieva, M. (2005). Betalain biosynthesis by red beet (Beta vulgaris L.) hairy root culture. Process Biochemistry, 40(5), 1531–1533.
Petkova, N., Vrancheva, R., Denev, P., Ivanov, I., & Pavlov, A. (2013). HPLC-RID method for determination of inulin and fructooligosaccharides. Conference: XI National Conference “Natural Sciences 2013, “Bishop Konstantin Preslavski” Shumen University, pp 99–107.
Perry, N. S. L., Houghton, P. J., Theobald, A., Jenner, P., & Perry, E. K. (2000). In vitro inhibition of human erythrocyte acetylcholinesterase by Salvia lavandulaefolia essential oil and constituent terpenes. Journal of Pharmacy and Pharmacology, 52(7), 895–902.
Georgiev, V., Marchev, A., Haas, C., Weber, J., Nikolova, M., Bley, T., & Pavlov, A. (2011). Production of oleanolic and ursolic acids by callus cultures of Salvia tomentosa Mill. Biotechnology and Biotechnological Equipment, 25(1), 34–38.
Georgiev, V., Ivanov, I., & Pavlov, A. (2010). Obtaining and selection of Pancratium maritimum L. In vitro cultures with acetylcholinesterase inhibitory action. Biotechnology and Biotechnological Equipment, 24, 149–153.
Wilson, S. A., & Roberts, S. C. (2012). Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnology Journal, 10(3), 49–268.
Weathers, P. J., Towler, M. J., & Xu, J. (2010). Bench to batch: advances in plant cell culture for producing useful products. Applied Microbiology and Biotechnology, 85(5), 1339–1351.
Ali, M., & Abbasi, B. H. (2014). Light-induced fluctuations in biomass accumulation, secondary metabolites production and antioxidant activity in cell suspension cultures of Artemisia absinthium L. Journal of Photochemistry and Photobiology B: Biology, 140, 223–227.
Zhao, J., Zhu, W. H., & Hu, O. (2001). Enhanced catharanthine production in catharanthus roseus cell cultures by combined elicitor treatment in shake flasks and bioreactors. Enzyme and Microbial Technology, 28, 673–681.
Tzin, V., & Galili, G. (2010). The biosynthetic pathways for shikimate and aromatic amino acids in Arabidopsis thaliana. In G. Jander (Ed.), The arabidopsis book (pp. 2–18). Minneapolis: The American Society of Plant Biologists.
Pavlov, A., Berkov, S., Weber, J., & Bley, T. (2009). Hyoscyamine biosynthesis in Datura stramonium hairy root in vitro systems with different ploidy levels. Applied Biochemistry and Biotechnology, 153, 210–225.
Sung, S. S., Kormanik, P. P., & Xu, P. D. (1989). Sucrose metabolic pathways in sweetgum and pecan seedlings. Tree Physiology, 5, 39–52.
Baena-Gonzalez, E., Rolland, F., Thevelein, J. M., & Sheen, J. (2007). A central integrator of transcription networks in plant stress and energy signalling. Nature, 448, 938–942.
Berkov, S., Bastida, J., Nikolova, M., Viladomat, F., & Codina, C. (2008). Rapid TLC/GC-MS identification of acetylcholinesterase inhibitors in alkaloid extracts. Phytochemical Analysis, 19(5), 411–419.
Acknowledgments
The authors thank for the financial support of this research by the Bulgarian Science Foundation, Bulgarian Ministry of Education and Science under contract number DMU 03/77 - 2011.
Conflict of Interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Georgieva, L., Ivanov, I., Marchev, A. et al. Protopine Production by Fumaria Cell Suspension Cultures: Effect of Light. Appl Biochem Biotechnol 176, 287–300 (2015). https://doi.org/10.1007/s12010-015-1574-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1574-6