Abstract
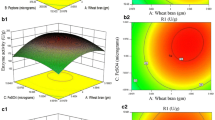
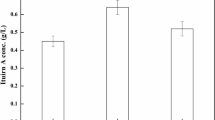
The thermotolerant yeast Pichia etchellsii produces multiple cell bound β-glucosidases that can be used for synthesis of important alkyl- and aryl-glucosides. Present work focuses on enhancement of β-glucosidase I (BGLI) production in Pichia pastoris. In the first step, one-factor-at-a-time experimentation was used to investigate the effect of aeration, antifoam addition, casamino acid addition, medium pH, methanol concentration, and mixed feed components on BGLI production. Among these, initial medium pH, methanol concentration, and mixed feed in the induction phase were found to affect BGLI production. A 3.3-fold improvement in β-glucosidase expression was obtained at pH 7.5 as compared to pH 6.0 on induction with 1 % methanol. Addition of sorbitol, a non-repressing substrate, led to further enhancement in β-glucosidase production by 1.4-fold at pH 7.5. These factors were optimized with response surface methodology using Box–Behnken design. Empirical model obtained was used to define the optimum “operating space” for fermentation which was a pH of 7.5, methanol concentration of 1.29 %, and sorbitol concentration of 1.28 %. Interaction of pH and sorbitol had maximum effect leading to the production of 4,400 IU/L. The conditions were validated in a 3-L bioreactor with accumulation of 88 g/L biomass and 2,560 IU/L β-glucosidase activity.





Similar content being viewed by others
Abbreviations
- BGLI:
-
β-glucosidase
- pNPG:
-
p-Nitrophenyl glucopyranoside
- OFAT:
-
One-factor-at-a-time
- RSM:
-
Response surface methodology
References
Baranwal, R., Jain, S., Shah, M. A., & Mishra, S. (2009). Elucidation of catalytically important residues in a large family 3 β-glucosidase from Pichia etchellsii. New Biotechnology, 25, Supplement, S126.
Barbosa, A. M., Giese, E. C., Dekker, R. F. H., Borsato, D., Briones Pérez, A. I., & Úbeda Iranzo, J. F. (2010). Extracellular β-glucosidase production by the yeast Debaromyces pseudopolymorphus UCLM-NS7A: optimization using response surface methodology. New Biotechnology, 27, 374–381.
Bhataya, A., Schmidt-Dannert, C., & Lee, P. C. (2009). Metabolic engineering of Pichia pastoris X-33 for lycopene production. Process Biochemistry, 44, 1095–1102.
Bhatia, Y., Mishra, S., & Bisaria, V. S. (2002). Microbial β-glucosidases: cloning, properties, and applications. Critical Reviews in Biotechnology, 22, 375–407.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Brierley, R. A., Davis, G. R., & Holtz, G. C. (1994). Production of insulin-like growth factor-1 in methylotrophic yeast cells. Google Patents.
Büchs, J. (2001). Introduction to advantages and problems of shaken cultures. Biochemical Engineering Journal, 7, 91–98.
Cereghino, J. L., & Cregg, J. M. (2000). Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiology Reviews, 24, 45–66.
Cos, O., Ramón, R., Montesinos, J., & Valero, F. (2006). Operational strategies, monitoring, and control of heterologous protein production in the methylotrophic yeast Pichia pastoris under different promoters: a review. Microbial Cell Factories, 5, 17.
Crout, D. H. G., & Vic, G. (1998). Glycosidases and glycosyl transferases in glycoside and oligosaccharide synthesis. Current Opinion in Chemical Biology, 2, 98–111.
Daly, R., & Hearn, M. T. (2005). Expression of heterologous proteins in Pichia pastoris: a useful experimental tool in protein engineering and production. Journal of Molecular Recognition, 18, 119–138.
Holmes, W., Darby, R., Wilks, M., Smith, R., & Bill, R. (2009). Developing a scalable model of recombinant protein yield from Pichia pastoris: the influence of culture conditions, biomass, and induction regime. Microbial Cell Factories, 8, 35.
Hu, S., Li, L., Qiao, J., Guo, Y., Cheng, L., & Liu, J. (2006). Codon optimization, expression, and characterization of an internalizing anti-ErbB2 single-chain antibody in Pichia pastoris. Protein Expression and Purification, 47, 249–257.
Inan, M., Chiruvolu, V., Eskridge, K. M., Vlasuk, G. P., Dickerson, K., Brown, S., & Meagher, M. M. (1999). Optimization of temperature–glycerol–pH conditions for a fed-batch fermentation process for recombinant hookworm Ancylostoma caninum anticoagulant peptide (AcAP-5) production by Pichia pastoris. Enzyme and Microbial Technology, 24, 438–445.
Iwashita, K., Todoroki, K., Kimura, H., Shimoi, H., & Ito, K. (1998). Purification and characterization of extracellular and cell wall bound beta-glucosidases from Aspergillus kawachii. Bioscience, Biotechnology, and Biochemistry, 62, 1938–1946.
Jafari, R., Sundström, B. E., & Holm, P. (2011). Optimization of production of the anti-keratin 8 single-chain Fv TS1-218 in Pichia pastoris using design of experiments. Microbial Cell Factories, 10, 1–8.
Jahic, M., Gustavsson, M., Jansen, A.-K., Martinelle, M., & Enfors, S.-O. (2003). Analysis and control of proteolysis of a fusion protein in Pichia pastoris fed-batch processes. Journal of Biotechnology, 102, 45–53.
Jahic, M., Veide, A., Charoenrat, T., Teeri, T., & Enfors, S. O. (2006). Process technology for production and recovery of heterologous proteins with Pichia pastoris. Biotechnology Progress, 22, 1465–1473.
Jin, H., Liu, G., Ye, X., Duan, Z., Li, Z., & Shi, Z. (2010). Enhanced porcine interferon-α production by recombinant Pichia pastoris with a combinational control strategy of low induction temperature and high dissolved oxygen concentration. Biochemical Engineering Journal, 52, 91–98.
Job, J., Sukumaran, R. K., & Jayachandran, K. (2010). Production of a highly glucose tolerant β-glucosidase by Paecilomyces variotii MG3: optimization of fermentation conditions using Plackett–Burman and Box–Behnken experimental designs. World Journal of Microbiology and Biotechnology, 26, 1385–1391.
Jungo, C., Schenk, J., Pasquier, M., Marison, I. W., & von Stockar, U. (2007). A quantitative analysis of the benefits of mixed feeds of sorbitol and methanol for the production of recombinant avidin with Pichia pastoris. Journal of Biotechnology, 131, 57–66.
Klöckner, W., & Büchs, J. (2012). Advances in shaking technologies. Trends Biotechnol, 30, 307–314.
Kobayashi, K., Kuwae, S., Ohya, T., Ohda, T., Ohyama, M., Ohi, H., Tomomitsu, K., & Ohmura, T. (2000). High-level expression of recombinant human serum albumin from the methylotrophic yeast Pichia pastoris with minimal protease production and activation. Journal of Bioscience and Bioengineering, 89, 55–61.
Lee, C. Y., Lee, S. J., Jung, K. H., Katoh, S., & Lee, E. K. (2003). High dissolved oxygen tension enhances heterologous protein expression by recombinant Pichia pastoris. Process Biochemistry, 38, 1147–1154.
Li, P., Anumanthan, A., Gao, X.-G., Ilangovan, K., Suzara, V. V., Düzgüneş, N., & Renugopalakrishnan, V. (2007). Expression of recombinant proteins in Pichia pastoris. Applied Biochemistry and Biotechnology, 142, 105–124.
Pandey, M., & Mishra, S. (1995). Cloning and expression of β-glucosidase gene from the yeast Pichia etchellsii. Journal of Fermentation and Bioengineering, 80, 446–453.
Pichia expression kit. Available from: www.invitrogen.com/content/sfs/manuals/pich_man.pdf .
Plackett, R. L., & Burman, J. P. (1946). The design of optimum multifactorial experiments. Biometrika, 33, 305–325.
Rather, M. Y., Mishra, S., & Aravinda, S. (2013). Exploring the synthetic potential of cell bound β-glycosidase of Pichia etchellsii. Journal of Biotechnology, 165, 63–68.
Rather, M. Y., Mishra, S., Verma, V., & Chand, S. (2012). Biotransformation of methyl-β-d-glucopyranoside to higher chain alkyl glucosides by cell bound β-glucosidase of Pichia etchellsii. Bioresource Technology, 107, 287–294.
Rols, J., & Goma, G. (1991). Enhanced oxygen transfer rates in fermentation using soybean oil-in-water dispersions. Biotechnology Letters, 13, 7–12.
Routledge, S. J., Hewitt, C. J., Bora, N., & Bill, R. M. (2011). Antifoam addition to shake flask cultures of recombinant Pichia pastoris increases yield. Microbial Cell Factories, 10, 17.
Sánchez, C. (2009). Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnology Advances, 27, 185–194.
Sreekrishna, K., Brankamp, R. G., Kropp, K. E., Blankenship, D. T., Tsay, J.-T., Smith, P. L., Wierschke, J. D., Subramaniam, A., & Birkenberger, L. A. (1997). Strategies for optimal synthesis and secretion of heterologous proteins in the methylotrophic yeast Pichia pastoris. Gene, 190, 55–62.
Stratton, J., Chiruvolu, V., & Meagher, M. (1998). in Pichia protocols, Springer, pp. 107–120.
Strobel, R., & Sullivan, G. (1999). Experimental design for improvement of fermentations. Manual of Industrial Microbiology Biotechnology, 2, 80–93.
Su, J.-H., Xu, J.-H., Lu, W.-Y., & Lin, G.-Q. (2006). Enzymatic transformation of ginsenoside Rg3 to Rh2 using newly isolated Fusarium proliferatum ECU2042. Journal of Molecular Catalysis B: Enzymatic, 38, 113–118.
Swalley, S. E., Fulghum, J. R., & Chambers, S. P. (2006). Screening factors affecting a response in soluble protein expression: formalized approach using design of experiments. Analytical Biochemistry, 351, 122–127.
Villatte, F., Hussein, A., Bachmann, T., & Schmid, R. (2001). Expression level of heterologous proteins in Pichia pastoris is influenced by flask design. Applied Microbiology and Biotechnology, 55, 463–465.
von Rybinski, W., & Hill, K. (1998). Alkyl polyglycosides—properties and applications of a new class of surfactants. Angewandte Chemie International Edition, 37, 1328–1345.
Wallecha, A., & Mishra, S. (2003). Purification and characterization of two β-glucosidases from a thermo-tolerant yeast Pichia etchellsii. Biochimica et Biophysica Acta (BBA)—Proteins & Proteomics, 1649, 74–84.
Wegner, E. H. (1983). Biochemical conversions by yeast fermentation at high cell densities. Google Patents.
Weuster-Botz, D. (2000). Experimental design for fermentation media development: Statistical design or global random search? Journal of Bioscience and Bioengineering, 90, 473–483.
Wu, D., Chu, J., Hao, Y.-Y., Wang, Y.-H., Zhuang, Y.-P., & Zhang, S.-L. (2012). Incomplete protein disulphide bond conformation and decreased protein expression result from high cell growth during heterologous protein expression in Pichia pastoris. Journal of Biotechnology, 157, 107–112.
Xie, J., Zhou, Q., Du, P., Gan, R., & Ye, Q. (2005). Use of different carbon sources in cultivation of recombinant Pichia pastoris for angiostatin production. Enzyme and Microbial Technology, 36, 210–216.
Acknowledgments
Financial assistance from the Department of Science and Technology, Government of India, New Delhi is gratefully acknowledged. Senior research fellowship to Miss Jyoti Batra from the Council of Scientific and Industrial Research, New Delhi and scholarship to Mr. Dhananjay Beri provided by the Ministry of Human Resource and Development are gratefully acknowledged. We also thank Mr. Ashwani Gautam and Mr. Anshul Sharma for assisting in running the fermentor.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 66 kb)
Rights and permissions
About this article
Cite this article
Batra, J., Beri, D. & Mishra, S. Response Surface Methodology Based Optimization of β-Glucosidase Production from Pichia pastoris . Appl Biochem Biotechnol 172, 380–393 (2014). https://doi.org/10.1007/s12010-013-0519-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0519-1