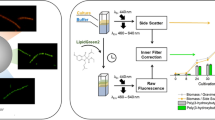
Abstract
Culture fluorescence measurement is an indirect and non-invasive method of biomass estimation to assess the metabolic state of the microorganism in a fermentation process. In the present investigation, NAD(P)H fluorescence has been used for on-line in situ characterization of metabolic changes occurring during different phases of batch cultivation of Azohydromonas australica in growth associated poly(3-hydroxybutyrate) or PHB production. A linear correlation between biomass concentration and net NAD(P)H fluorescence was obtained during early log phase (3–12 h) and late log phase (24–39 h) of PHB fermentation. After 12 h (mid log phase) cultivation PHB accumulation shot up and a drop in culture fluorescence was observed which synchronously exhibited continuous utilization of NAD(P)H for the synthesis of biomass and PHB formation simultaneously. A decrease in the observed net fluorescence value was observed again towards the end of fermentation (at 39 h) which corresponded very well with the culture starvation and substrate depletion towards the end of cultivation inside the bioreactor. It was therefore concluded that NAD(P)H fluorescence measurements could be used for indication of the time of fresh nutrient (substrate) feed during substrate limitation to further enhance the PHB production.





Similar content being viewed by others
References
Lee, S. Y. (1996). Bacterial polyhydroxyalkanoates. Biotechnology and Bioengineering, 49, 1–14.
Loo, C. Y., & Sudesh, K. (2007). Polyhydroxyalkanoates: bio-based microbial plastics and their properties. Malaysian Polymer Journal, 2, 31–57.
Tokiwa, Y., & Ugwu, C. U. (2007). Biotechnological production of (R)-3-hydroxybutyric acid monomer. Journal of Biotechnology, 132, 264–272.
Zinn, M., Witholt, B., & Egli, T. (2001). Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Advanced Drug Delivery Reviews, 53, 5–21.
Khanna, S., & Srivastava, A. K. (2005). Recent advances in microbial polyhydroxyalkanoates. Process Biochemistry, 40, 607–619.
Choi, J., & Lee, S. Y. (1999). Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Applied Microbiology and Biotechnology, 51, 13–21.
Braunegg, G., & Bogensberger, B. (1985). Zur Kinetik des Wachstums und der Speicherung von Poly-d(−)-3-hydroxybuttersäure bei Alcaligenes latus. Acta Biotechnologica, 5, 339–345.
Wang, F., & Lee, S. Y. (1997). Poly (3-hydroxybutyrate) production with high productivity and high polymer content by a fed-batch culture of Alcaligenes latus under nitrogen limitation. Applied and Environmental Microbiology, 63, 3703–3706.
Genser, K. F., Renner, G., & Schwab, H. (1998). Molecular cloning, sequencing and expression in Escherichia coli of the poly(3-hydroxyalkanoate) synthesis genes from Alcaligenes latus DSM1124. Journal of Biotechnology, 64, 123–135.
Yezza, A., Halasz, A., Levadoux, W., & Hawari, J. (2007). Production of poly-β-hydroxybutyrate (PHB) by Alcaligenes latus from maple sap. Applied Microbiology and Biotechnology, 77, 269–274.
Liden, G. (1993). In situ fluorescence measurements—clarifying or blurring the picture? Pure and Applied Chemistry, 65, 1927–1932.
Ju, L. K., Chen, F., & Xia, Q. (2005). Monitoring microaerobic denitrification of Pseudomonas aeruginosa by online NAD(P)H fluorescence. Journal of Industrial Microbiology and Biotechnology, 32, 622–628.
Duysens, L. N. M., & Amesz, J. (1957). Fluorescence spectrophotometry of reduced phosphopyridine nucleotide in intact cells in the near-ultraviolet and visible region. Biochimica et Biophysica Acta, 24, 19–26.
Lee, D., Song, S. H., Kim, J. H., & Yoo, Y. J. (2002). On-line monitoring of the denitrification process by measurement of NADH fluorescence. Biotechnology Letters, 24, 949–952.
Srivastava, S., Harsh, S., & Srivastava, A. K. (2008). Use of NADH fluorescence measurement for on-line biomass estimation and characterization of metabolic status in bioreactor cultivation of plant cells for azadirachtin (a biopesticide) production. Process Biochemistry, 43, 1121–1123.
Stärk, E., Hitzmann, B., Schügerl, K., Scheper, T., Fuchs, C., Köster, D., et al. (2002). In-situ-fluorescence-probes: a useful tool for non-invasive bioprocess monitoring. Advances in Biochemical Engineering/Biotechnology, 74, 21–38.
Gahlawat, G., & Srivastava, A. K. (2012). Estimation of fundamental kinetic parameters of polyhydroxybutyrate fermentation process of Azohydromonas australica using statistical approach of media optimization. Applied Biochemistry and Biotechnology, 168, 1051–1064.
Beyeler, W., Einsele, A., & Fiechter, A. (1981). On-line measurements of culture fluorescence: method and application. European Journal of Applied Microbiology and Biotechnology, 13, 10–14.
Luong, J. H. T., & Carrier, D. J. (1986). On-line measurement of culture fluorescence during cultivation of Methylomonas mucosa. Applied Microbiology and Biotechnology, 24, 65–70.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426–428.
Riis, V., & Mai, W. (1988). Gas chromatographic determination of poly-β-hydroxybutyric acid in microbial biomass after hydrochloric acid propanolysis. Journal of Chromatography A, 445, 285–289.
Grothe, E., & Chisti, Y. (2000). Poly (β-hydroxybutyric acid) thermoplastic production by Alcaligenes latus: behavior of fed-batch cultures. Bioprocess Engineering, 22, 441–449.
Grothe, E., Moo-Young, M., & Chisti, Y. (1999). Fermentation optimization for the production of poly (β-hydroxybutyric acid) microbial thermoplastic. Enzyme and Microbial Technology, 25, 132–141.
Hrabak, O. (1992). Industrial production of Poly-β-hydroxybutyrate. FEMS Microbiology Reviews, 103, 251–256.
Reardon, K. F., Scheper, T. H., & Bailey, J. E. (1987). Metabolic pathway rates and culture fluorescence in batch fermentations of Clostridium acetobutylicum. Biotechnology Progress, 3, 153–167.
Srivastava, A. K., & Volesky, B. (1991). On-line fluorescence measurements in assessing culture metabolic acitivities. Applied Microbiology and Biotechnology, 34, 450–457.
Shin, H. D., Oh, D. H., Jung, Y. M., Ghim, S. Y., & Lee, Y. H. (2002). Comparison of phbC genes cloned from Ralstonia eutropha and Alcaligenes latus for utilization in metabolic engineering of polyhydroxyalkanoate biosynthesis. Biotechnology Letters, 24, 539–545.
Page, W. J., Tindale, A., Chandra, M., & Kwon, E. (2001). Alginate formation in Azotobacter vinelandii UWD during stationary phase and the turnover of poly-β-hydroxybutyrate. Microbiology, 147, 483–490.
Zabriskie, D. W., & Humphrey, A. E. (1978). Estimation of fermentation biomass concentration by measuring culture fluorescence. Applied and Environmental Microbiology, 35, 337–343.
Chattopadhyay, S., Bisaria, V. S., Scheper, T., & Srivastava, A. (2002). Non-invasive methods for determination of cellular growth in Podophyllum hexandrum suspension cultures. Biotechnology and Bioprocess Engineering, 7, 331–334.
Acknowledgment
The Senior Research Fellowship (SRF) award by The Department of Biotechnology (DBT), Govt of India, New Delhi, for the execution of the project is gratefully acknowledged by one of the authors (Ms Geeta Gahlawat).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gahlawat, G., Srivastava, A.K. Use of NAD(P)H Fluorescence Measurement for On-Line Monitoring of Metabolic State of Azohydromonas australica in Poly(3-hydroxybutyrate) Production. Appl Biochem Biotechnol 169, 821–831 (2013). https://doi.org/10.1007/s12010-012-0040-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-0040-y